WSC 22-23
Conference 11
Case IV:
Signalment:
9-year-old, female, Crawl Cay Boa (Boa imperator)
History:
This animal was the only affected animal from a small, multi-species, zoological collection which presented with anorexia for the preceding 7 months, with gradually progressive weight loss resulting in a thin body condition at presentation.
Gross Pathology:
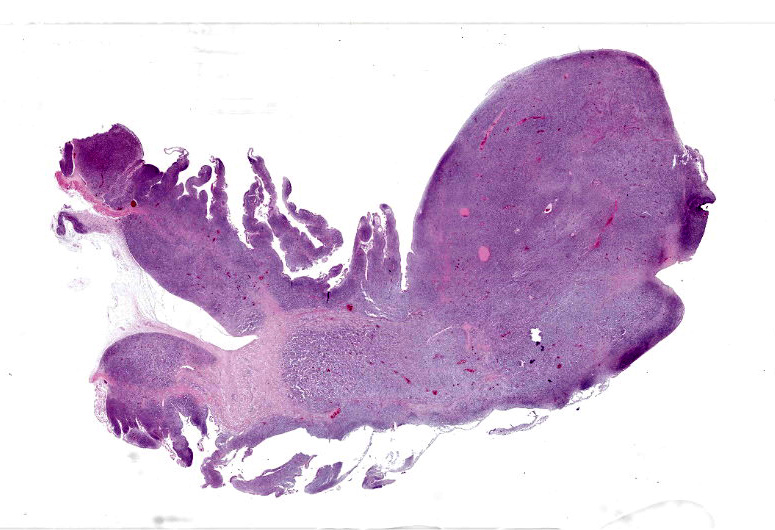
There was a 20cm x 10cm fluid filled structure within distended the mid-coelomic cavity which corresponded to an approximately 20cm long segment of jejunum which was markedly dilated up to approximately 10cm diameter. At this site, on the intestinal mucosal surface were numerous, white, raised nodules measuring up to approximately 1mm x 5mm which were interspersed by multifocal, firm, larger masses measuring up to 2cm diameter and forming multifocal papilliform projection which protruded into the intestinal lumen. At the aboral end of this segmental dilation there was an approximately 10 cm long segment of the intestine which was distorted and had ‘telescoped’ into the more distal intestine, forming an intussusception.
Laboratory Results:
Immunohistochemistry: CD3 negative, CD20 negative and Iba-1 positive. In-situ hybridisation: Boa CD20 negative.
Microscopic Description:
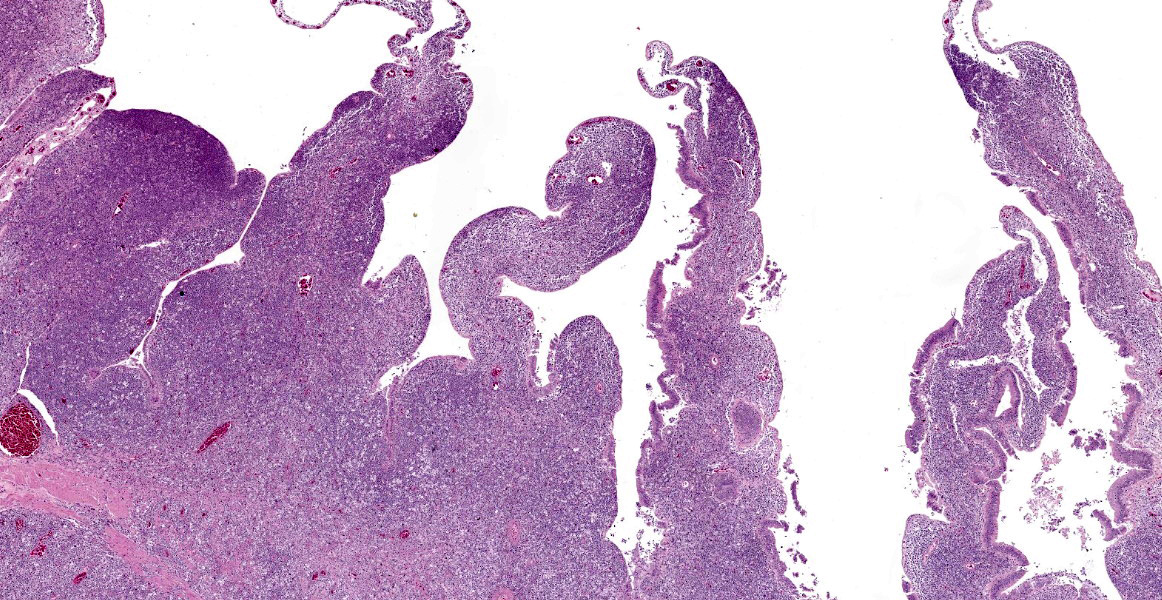
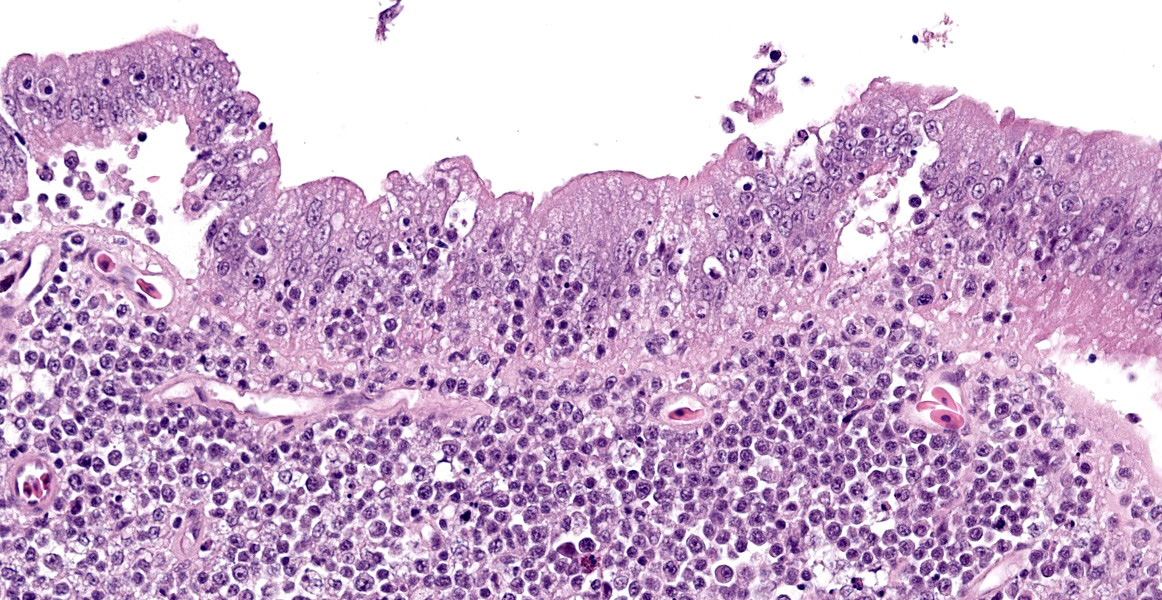
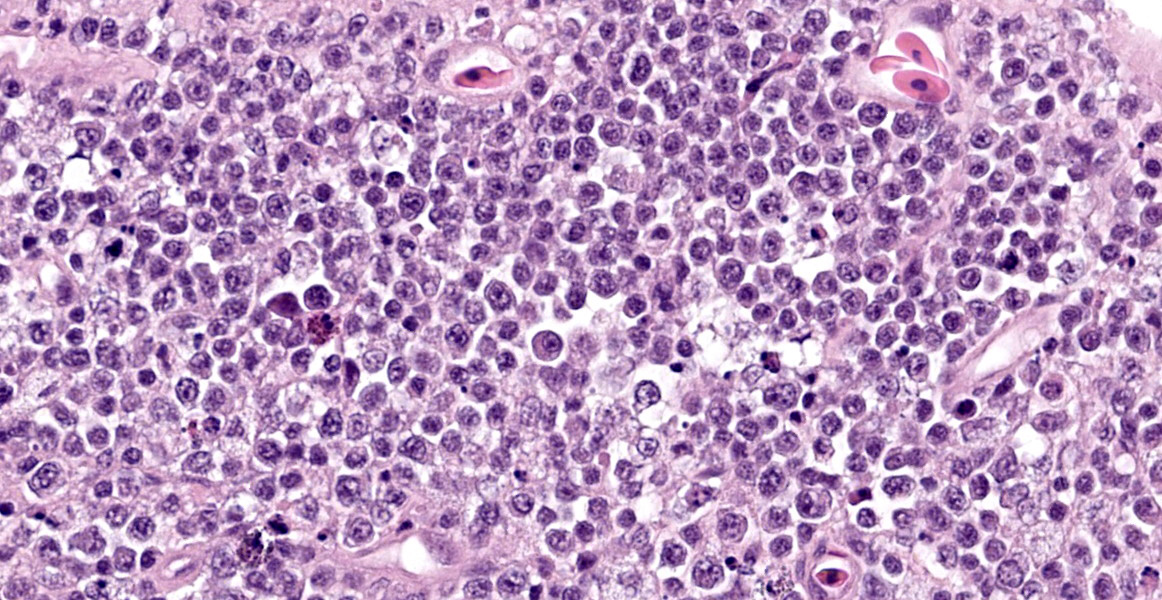
The intestine at the level of the intussusception was markedly expanded and transmurally effaced by an expansile, multilobulated, unencapsulated and poorly demarcated proliferation of neoplastic, monomorphic round cells forming sheets supported by a fine fibrovascular stroma. These variably formed frond like projections, coalescent polypoid masses or locally expanded the lamina propria. These cells were up to approximately 25 µm diameter (3-4 x the diameter of an erythrocyte), with distinct borders and a large amount of eosinophilic cytoplasm. The nuclei were round to oval, central to eccentrically positioned, with euchromatic and stippled to vesicular chromatin and frequent large nucleoli. There was moderate anisocytosis and anisokaryosis with 10 mitotic figures per 2.37 mm² (equivalent to 10 high power fields; hpf; x400 magnification), some of which were bizarre in form. Occasionally, in multifocal areas, these cells were expanded by increased, clear intercellular spacing (intercellular oedema). The epithelium was multifocally ulcerated with replacement by eosinophilic, homogenous material (fibrin), degenerate heterophils, sloughed necrotic epithelial cells, eosinophilic and karyorrhectic debris, and swathes of basophilic. In other areas, there was slight piling up of epithelial nuclei (hyperplasia; attempted regeneration).
Contributor’s Morphologic Diagnoses:
Intestinal histiocytic sarcoma with secondary intussusception.
Contributor’s Comment:
The features in this consisted of a coelomic dilation corresponding to a large intestinal dilation and associated intussusception. Microscopic examination revealed extensive effacement of the intestinal mucosa by sheets of neoplastic round cells, which were strongly Iba-1 positive, indicative of a histiocytic sarcoma at this site. Considering the lack of additional lesions, this intussusception was most likely secondary to this neoplasm.
Intestinal intussusception is clinically defined as the telescoping of one segment of digestive tract into the lumen of an adjacent segment, forming an inner intussusceptum and surrounding intussuscipiens. This forms a distended segment of intestine, with significant vascular compression resulting in progression to ischaemic necrosis of both the intussusceptum and intussuscipiens.
Histiocytic sarcoma itself has only been rarely reported in boa species, with a single case identified in both a rosy boa (Lichanura trivirgata) and a boa constrictor (Boa constrictor), whilst an additional single histiocytoma was identified in a rainbow boa (Epicrates cenchria).4,5 Similarly, histiocytic neoplasms have been only rarely identified in other snake species including single reports in a common garter snake (Thamnophis sirtalis) and bull snake (Pituophis catenifer sayi), though it was not stated in these cases if any additional confirmation such as immunohistochemistry was performed.5,15
Only a small number of published case reports have described intussusception secondary to other neoplasms in snakes, including T-cell lymphoma and myeloid leukaemia in boa constrictors and a non-characterised round cell tumor in an Australian sea snake (Hydrophis major).6,8,13 Similarly, only small numbers of published reports describe non-neoplastic causes of intussusception in snakes, including idiopathic intussusception in a pine snake (Pituophis melanoleucus) and intussusception secondary to cryptosporidiosis in a corn snake (Pantherophis guttatus).1,16
Differentiation of histiocytic tumors from other round cell lineages is often challenging in snakes, particularly in comparison to lymphoid proliferations, which often share similar morphological features.5 Furthermore, consistent immunohistological phenotyping of neoplasms is challenging in reptiles due to the lack of established antibody markers, preventing accurate and reliable immunophenotyping.13
Intussusception is a rare but recognized complication of round cell tumors in other species, including humans.2,9 Similarly to veterinary species, there are only limited individual case reports in human medical literature of histiocytic sarcoma associated intussusception.12
Contributing Institution:
Easter Bush Pathology - Royal (Dick) School of Veterinary Studies. https://www.ed.ac.uk/vet/services/easter-bush-pathology
JPC Diagnosis:
Intestine: Lymphoma.
JPC Comment:
While a less specific diagnosis of round cell tumor was considered, conference participants ultimately favored a diagnosis of lymphoma based on the histologic appearance of neoplastic cells which had scant cytoplasm and a generally round nucleus. Immunohistochemical evaluation conducted by the JPC was consistent with B-cell lymphoma. Most neoplastic cells had strong neoplastic immunoreactivity for PAX-5, a B cell marker, with few infiltrating T cells positive for CD-3 and IBA-1 highlighting few cells with dendritic morphology, consistent with tumor associated macrophages. Dr. LaDouceur, this week’s moderator and author of the Reptile Neoplasia chapter in Noninfectious Diseases and Pathology of Reptiles, explained that successful B cell markers are difficult to find in reptiles, and PAX-5 and BLA-36 provide the most utility. The presence of few cells with basophilic cytoplasmic granules lead to brief consideration for a metastatic chromatophoroma; however, there was metachromatic staining to the granules on Giemsa, identifying the cells as mast cells and likely part of inflammation secondary to mucosal ulceration.
In several studies of neoplasms in snakes, malignancies far outnumber benign tumors, ranging from 70 to 80% of all diagnosed neoplasms.3,10,15 In general, hematopoietic neoplasia, specifically lymphoma, is the most common neoplasm in snakes, though there is some variation between reports, presumedly due to variation in species composition in various collections.7,10,15 As in other species, lymphoma in snakes may be multicentric and form discrete masses or diffuse organomegaly with or without a leukemic component.
The histomorphology may appear plasmacytoid or histiocytoid, making immunohistochemical often necessary to confirm an uncommon diagnosis of histiocytic sarcoma.7 Other common neoplasms in snakes include soft tissue sarcomas, renal adenocarcinomas, fibrosarcomas, and melanomas.5
Another differential to consider for cases of histiocytic or granulomatous inflammation in snakes is atypical or nontuberculous Mycobacterium infection, which causes histiocytic, granulomatous, or occasionally heterophilic inflammation in snakes.11,14 Estimates of the prevalence of Mycobacterium in snake collections vary based on management practices from 0.1% to up to 30%.11 Mycobacterial infections cause diffuse or multifocal inflammatory infiltrates which can appear as gray to white masses in the subcutaneous tissue and visceral organs, with the pulmonary system most commonly affected.11 Acid fast staining, such as Ziehl-Neelson and Fite Farraco, can be used to identify the organisms, which may be rare or abundant, though occasionally the bacteria can be visualized on H&E or Gram staining.14
References:
- Bercier M, Zoll W, Rosenberg JF, et al. Gastric intussusceptions in a red corn snake (Pantherophis guttatus) associated with cryptosporidiosis. Case Reports Vet Med. 2017; 1-5.
- Bussell HR, Kroiss S, Tharakan SJ, Meuli M, Moehrlen U. Intussusception in children: lessons learned from intestinal lymphoma as a rare lead-point. Pediatr Surg Int. 2019;35(8):879-885.
- Catao-Dias JL, Nichols DK. Neoplasia in snakes at the National Zoological Park, Washington, DC (1978-1997). J Comp Pathol. 1999; 1290(1): 89-95.
- Duke EG, Harrison SH, Moresco A, et al. A Multi-Institutional Collaboration to Understand Neoplasia, Treatment and Survival of Snakes. Animals (Basel). 2022;12(3):258-275.
- Garner MM, Hernandez-Divers SM, Raymond JT. Reptile neoplasia: a retrospective study of case submissions to a specialty diagnostic service. Vet Clin Exot Anim. 2004; 7:653-671.
- Gillett AK, Ploeg R, Flint M, Mills PC. Postmortem examination of Australian sea snakes (Hydrophiinae): Anatomy and common pathologic conditions. Jour Vet Diagn Invest. 2017; 29:593-611.
- LaDouceur EEB. Reptile Neoplasia. In: Garner MM, Jacobson ER, eds. Noninfectious Diseases and Pathology of Reptiles. Boca Raton, FL: CRC Press. 2020:16-17.
- Máté LK, Simard J, Ducatelle R, Hellebuyck T. Ileocolic Intussusception Associated with a Multicentric Round Cell Tumor in a Red-Tailed Boa (Boa constrictor constrictor). J Herpetol Med Surg. 2021;32(1):11-19.
- Özant A, Arslan K, Özçay N, Besim H. Adult multicentric burkitt lymphoma with bowel obstruction due to intussusception. Turkish J Gastroenterol. 2018;29(3):361-364.
- Page-Karjian A, Hahne m, Leach K, et al. Neoplasia in Snakes at Zoo Atlanta during 1992-2012. Jour Zoo Wildl Medicine. 2017; 48(2): 521-524.
- Page-Karjian A, Knowles S, Howerth EW, et al. Pathology in Practice. Jour Am Vet Med Assoc. 2012; 241(9): 1159-1161.
- Speelman A, Kolbe J. A case report of histiocytic sarcoma of the hepatic flexure: peer reviewed case report. South African Radiogr. 2012;50(1):27-29.
- Summa NM, Guzman DS-M, Hawkins MG, et al. Tracheal and Colonic Resection and Anastomosis in a Boa Constrictor (Boa constrictor) with T-Cell Lymphoma. J Herpetol Med Surg. 2015;25(3-4):87.
- Stacy BA, Pessier AP, Ossiboff RJ. Host Response to Infectious Agents and Identification of Pathogens in Tissue Sections. In: Jacobson ER, Garner MM, eds. Infectious Diseases and Pathology of Reptiles. Volume I. 2nd Boca Raton, FL: CRC Press. 2021: 383.
- Sykes IV JM, Trupkiewicz JG. Reptile neoplasia at the Philadelphia Zoological Garden, 1901-2002. J Zoo Wildl Med. 2006;37(1):11-19.
- Wosar MA, Lewbart GA. Ileocolic intussusception in a pine snake (Pituophis melanoleucus). Vet Rec. 2006;158(20):698-699.