Joint Pathology Center
Veterinary Pathology Services
nesday Slide Conference
2017-2018
Conference 21
April 11th, 2018
CASE II: F1755364 (JPC 4101079).
Signalment: 18-year-old, male, castrated, domestic shorthair (Felis catus), feline.
History: An 18-year old male castrated domestic short-haired cat presented acutely non-responsive to the emergency veterinarian. The cat had an approximately 1 year history of intermittent neurologic signs including ataxia, right-sided head tilt, paresis and paralysis, as well as progressive weight loss. Previous diagnoses included chronic kidney disease and systemic hypertension (180-220mmHg systolic). Fundic evaluation at a prior examination revealed microhemorrhages and partial retinal detachment. The cat was euthanized due to poor prognosis.
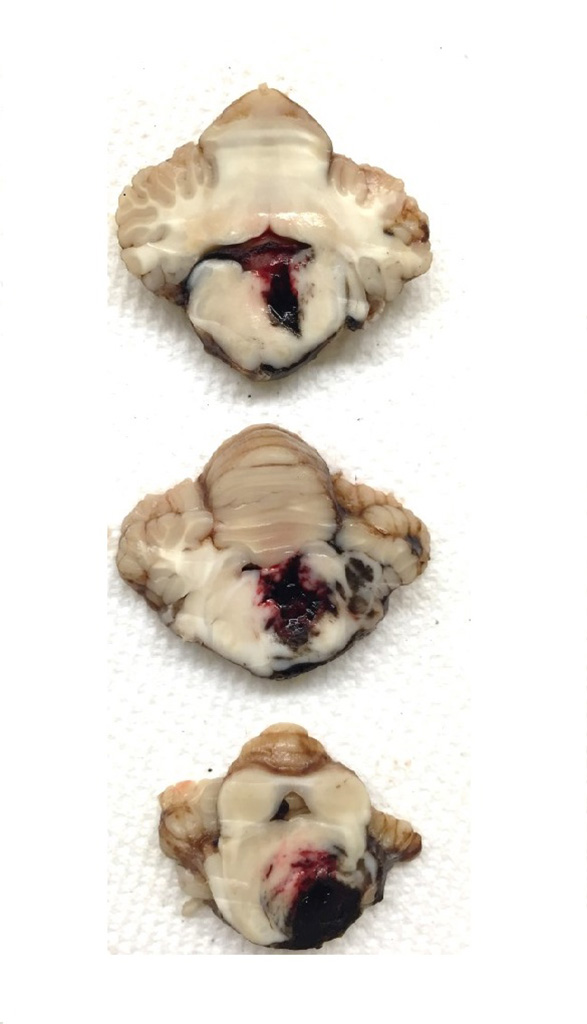
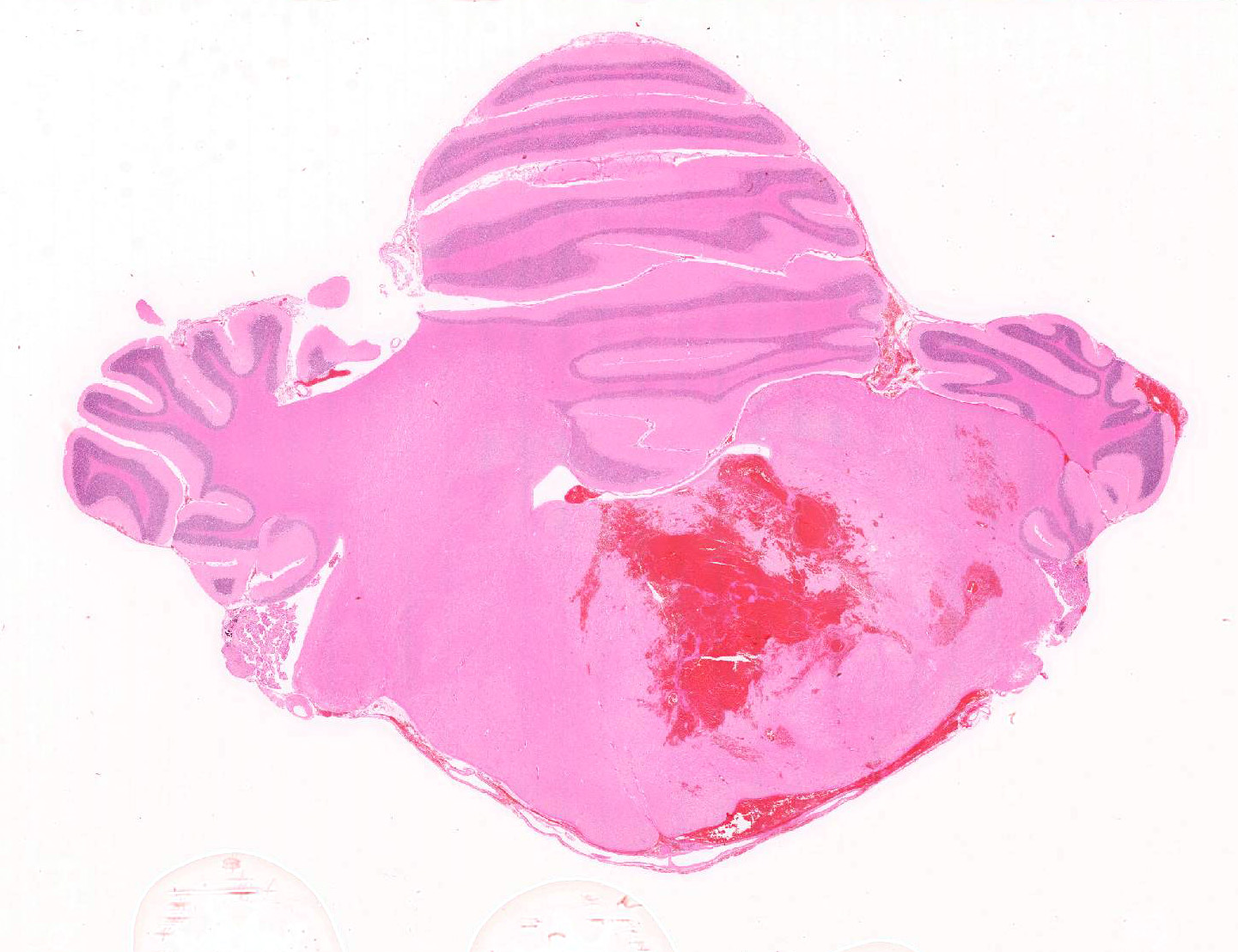
Gross Pathology: Extending from the left mid-thalamus caudally through the brainstem to the level of the obex is a poorly demarcated 4 x 1.5 x 1.0 cm region of hemorrhage with softening of the neural parenchyma (malacia) (Figure 1). Hemorrhage extends along the leptomeninges of the cerebellum, caudal cortex, and ventral brainstem. The ventral cervical and lumbar spinal cord has multifocal linear dark red foci on midline, centered on the ventral spinal artery.
Bilaterally, the kidneys are small (half normal size), pale, and firm, with diffusely pitted capsular surface that correlates with linear white streaks of fibrosis radiating from the medulla to the cortical surface.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.):
Clinical Pathology: BUN 54 mG/dL (normal 18-35 mG/dL); Creatinine 3.6 mG/dL (normal 0.8-2.4 mG/dL); urine specific gravity: 1.015.
Microscopic Description:
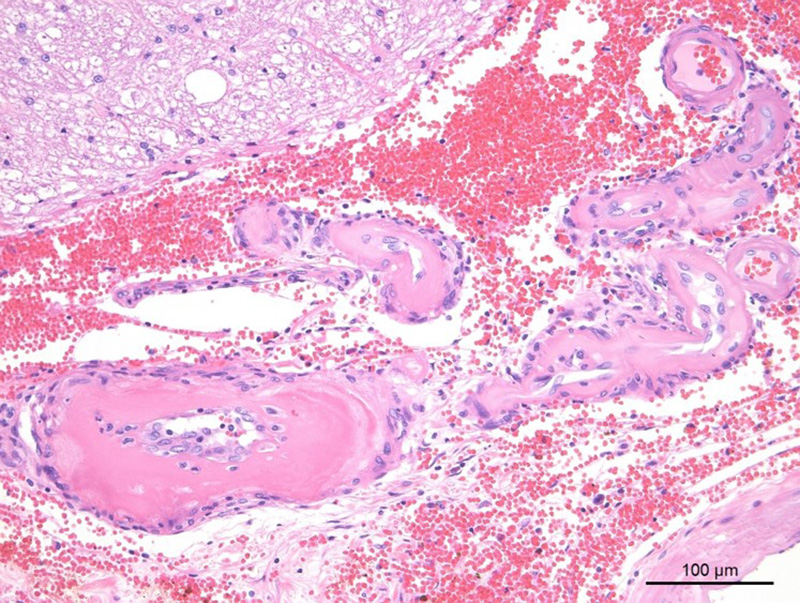
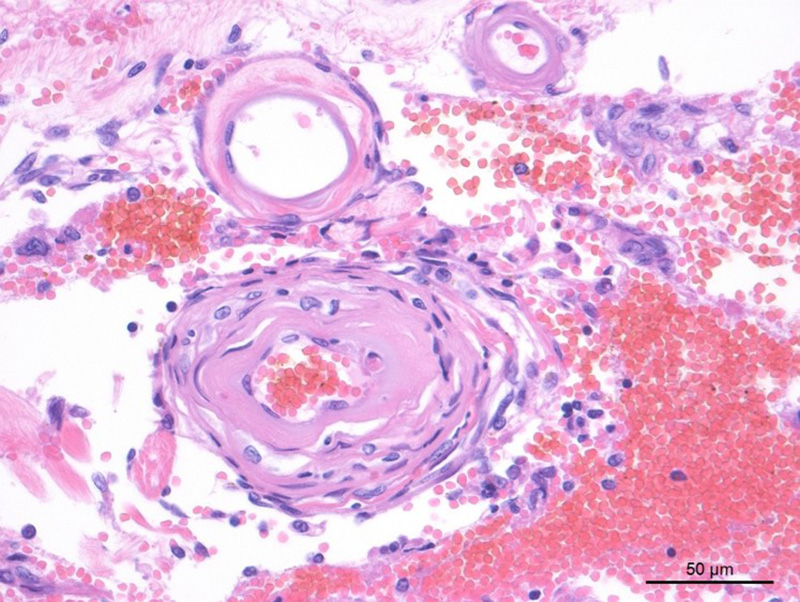
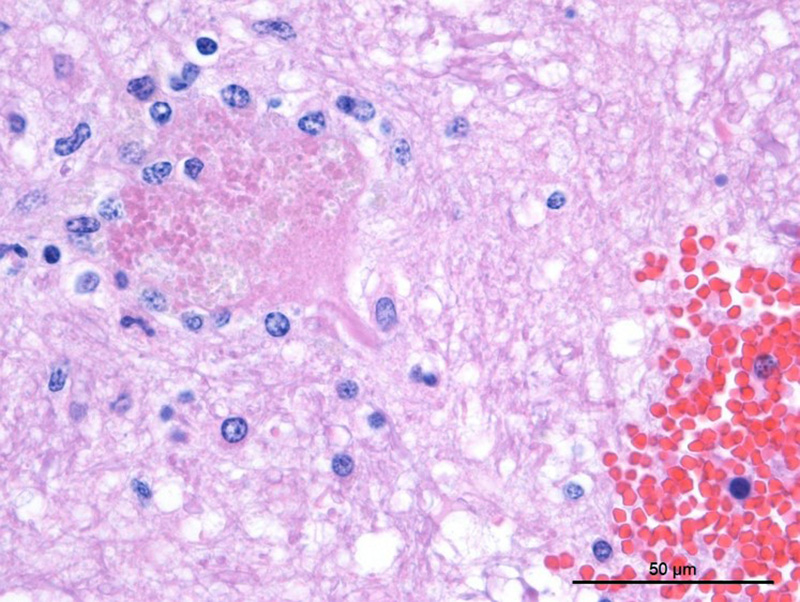
Cerebellum/brainstem: Multifocal meningeal and parenchymal arteries and arterioles are segmentally to diffusely thickened by bright eosinophilic amorphous, hyalinized, and occasionally granular material admixed with rare mural karyorrhectic debris (hyaline degeneration to fibrinoid necrosis). Endothelial cells are often plump with large nuclei and open chromatin (reactive) or vacuolated (degeneration). Multifocal vessels within the ventral leptomeninges and central brainstem are disrupted and replaced by severe regionally extensive hemorrhage partially organized by fibrin strands. Hemorrhage focally effaces approximately 40% of the brainstem cross-sectional area and tracks along the leptomeninges. Neuropil abutting the regions of hemorrhage is moderately vacuolated with edema and infiltrated by minimally increased numbers of glial cells. Multifocal neurons contain finely granular golden brown cytoplasmic lipofuscin pigment. There is rare mild meningeal and choroidal mineralization.
Spinal cord (not shown): Elsewhere along the spinal cord there were several similar sites of hemorrhage, each associated with similar vascular changes as those previously described. Some of these sites were more subacute to chronic with deposits of hematoidin and a more developed parenchymal reaction.
Kidney (not shown): The capsule is bossellated with multifocal depressions that correspond to radiating regions of marked interstitial fibrosis which dissect, separate, and replace approximately 40% of cortical tubules and glomeruli. Concentric perivascular, periglomerular, and peritubular fibrosis is frequent. High numbers of lymphocytes and plasma cells infiltrate the fibrous connective tissue. Glomeruli are often shrunken and sclerotic and Bowman’s capsules are moderately to markedly thickened and occasionally lined by plump reactive parietal cells. Tubules have moderately to severely thickened basement membranes and exhibit one or more of the following changes: tubular ectasia lined by attenuated epithelium, swollen vacuolated epithelium (degeneration), plump slightly basophilic epithelial lining (regeneration), or rare individual necrotic epithelial cells with shrunken cell borders, hypereosinophilic cytoplasm, and pyknotic nuclei. Tubules often contain proteinaceous fluid, occasional mineral or rare refractile crystals.
Contributor’s Morphologic Diagnosis:
Brainstem, cerebellum and spinal cord, arterioles: Severe chronic hyaline degeneration and fibrinoid necrosis with acute severe perivascular hemorrhage and necrosis (malacia).
Kidney (not shown): Severe chronic tubulointerstitial nephritis with marked interstitial fibrosis, glomerulosclerosis, and tubular proteinosis.
Contributor’s Comment: Hyaline degeneration of arteries is a non-specific pathologic change that refers to loss of normal cellular structure and deposition of amorphous material of the intima and media of a muscular artery or arteriole. The hyalinized appearance is a result of deposition of plasma protein, amyloid deposits, and/or necrosis of vascular smooth muscle.5 Hyaline degeneration can be associated with hypertension, uremia, diabetes mellitus, aging, hepatosis dietetica, organomercurial poisoning, mulberry heart disease, and cerebrospinal angiopathy/edema disease (among others).4,5
In this case, vascular changes are secondary to persistent hypertension (hypertensive vasculopathy) and chronic kidney disease. Other conditions that may result in secondary hypertension include: hyperthyroidism, diabetes mellitus, hyperaldosteronism, pheochromocytoma, chronic anemia (cats), hypothyroidism (dogs), erythropoietin therapy, and acute and chronic laminitis (cattle, horses).5 Hypertension can also be essential (primary). Essential hypertension is characterized by an increase in total peripheral vascular resistance due to a primary decrease in lumen diameter and increase in media thickness.
Renal disease is a common cause of hypertension in dogs and cats and contributed to the systemic hypertension identified in this case. Renal disease can be both a cause and effect of hypertension, complicating the pathogenesis in individual cases. Chronic renal disease results in impaired sodium and water excretion and thus increased blood volume. Furthermore, a hypertension-induced decrease in renal perfusion activates the renin-angiotensin-aldosterone system, which also results in increased blood pressure. Impaired renal perfusion and progressive vascular injury further exacerbates chronic kidney disease.
A common clinical presentation of animals with hypertensive vasculopathy is acute blindness secondary to retinal arterial degeneration with associated retinal vascular tortuosity, intraocular hemorrhage, and retinal detachment (hypertensive retinopathy).3 In cats and people a similar manifestation involving arteries of the central nervous system (hypertensive encephalopathy) has also been rarely described.1 Under normal conditions the vascular tone of cerebral arteries and arterioles are tightly regulated to maintain constant and appropriate perfusion of the brain. When systemic blood pressure reaches the upper limit of the capacity of cerebral autoregulation, the cerebral arterioles segmentally constrict and dilate. The appropriate autoregulatory response is maintained in constricted regions. In dilated regions, however, vascular overdistension disrupts endothelial tight junctions, allows leakage of plasma proteins into the extracellular space (vasogenic edema), activates endothelial cells into increase expression of adhesion molecules (ICAM-1, PCAM-1) and cytokines (IL-1, IL-6, IL-8, TNF-α), induces neutrophil and monocyte adhesion and migration, and over time results in repetitive injury to the endothelium and vascular wall with subsequent vascular degeneration, necrosis and hemorrhage.1,7 This case exhibits an end-stage response to chronic vascular damage as a result of persistent systemic hypertension. The intermittent nature of neurologic signs is consistent with repetitive small hemorrhages within the central nervous system and subsequent resolution. In fact, several other sites of more chronic hemorrhage were identified histologically in this cat’s spinal cord. These had deposition of hematoidin and a more developed tissue reaction. A final massive hemorrhage within the brainstem ultimately yielded the patient non-responsive.
JPC Diagnosis: Brainstem, arteries: Hyaline vascular necrosis, multifocal, moderate with perivascular fibrosis, multifocal, severe, acute parenchymal and meningeal hemorrhage, domestic shorthair (Felis catus), feline.
Conference Comment: There are three key terms regarding arterial degeneration to bear in mind: arteriosclerosis, atherosclerosis, and arteriolosclerosis.2
Arteriosclerosis (from the Greek, arterio- for artery and -sclerosis for hardening) is a chronic change consisting of: lumen narrowing, hardening, and loss of elasticity. Arteriosclerosis most commonly occurs in the abdominal aorta and points of arterial branching in older horses, ruminants, and carnivores secondary to a proliferative and degenerative change rather than inflammatory. A key feature is the lack of lipid deposition, which is a feature of atherosclerosis. The pathogenesis of plaque formation has not been fully explained but there are two main theories: (1) platelet microthrombi form in areas of turbulence or endothelial damage leading to release of platelet-derived growth factor (PDGF) and transforming growth factor-β, the former resulting in migration and proliferation of smooth muscle cells; and (2) the damage to the endothelium results in the endothelial cells themselves producing mitogens like PDGF. In reality, the pathogenesis is most likely a combination of the two. Microscopically, those mitogens cause migration of smooth muscle cells (or myointimal cells) from the media to the intima which act similarly to fibroblasts surrounding arteries by producing the matrix that forms the plaque composed of: collagen, elastic tissue, and proteoglycans.6
Atherosclerosis, which is characterized by the formation of an atheroma, a focal, raised, intimal fibrofatty plaque composed of cholesterol esters, is most common in humans and is only applicable to domestic animals in relation to animal models of human disease. Some animals are susceptible to the formation of atheromas (rabbits, chickens, and pigs) and are thus, good animal models for research purposes. Dogs, cats, cattle, goats, and rats, on the other hand, are atheroresistant (with few exceptions, see below). In general, pigs and non-human primates are the most widely used large-animal models and variations of genetically modified mice are becoming available as well. Clinically, atherosclerosis in humans often results in myocardial infarction, stroke, and peripheral vascular resistance. More common in domestic animals are fatty streaks, another type of intimal lesion, which are often found in the aorta and larger arteries of ruminants and swine. These appear grossly as soft, smooth, flat lesions of varying sizes that are stained bright orange with Sudan IV. There is no known correlation of fatty streaks with the formation of atherosclerotic plaques. The cause of atherosclerosis is multifactorial and explained by the “response to injury” hypothesis which states that endothelial injury or dysfunction can result from hyperlipidemia (mainly cholesterol from low-density lipoprotein and very-low-density lipoprotein). The following steps (platelet adhesion and smooth muscle migration and proliferation) are the same as for arteriosclerosis. As mentioned above, the key microscopic feature differentiating the two is the presence of lipid, which can be extracellular or intracellular (within activated macrophages or smooth muscle cells) called “foam cells”. In fatty streaks, lipid in activated macrophages is most prevalent and in atherosclerosis, lipid in smooth muscle cells predominates. In pigs, atheromas most frequently form in the aorta and extramural coronary arteries, cerebral and iliac arteries. The main predisposing factor is a diet containing excess cholesterol and the plaques, unlike humans, rarely lead to fully occlusive thrombus formation. Dogs, although generally atheroresistant, can develop atherosclerosis secondary to hypercholesterolemia from hypothyroidism or diabetes mellitus. Additionally, Miniature Schnauzers have a genetic predisposition towards idiopathic hyperlipoproteinemia which often results in atherosclerosis. Microscopically, in dogs the lipid accumulates in the tunica media, whereas, in humans, it is present in the intima. As an aside, historically the term “xanthomatosis” has been used to describe this condition in animals. Current literature dictates that xanthomas are accumulation soft lipid-laden foam cells in the subcutaneous and cutaneous tissues only, and is a well-known condition in cats deficient in lipoprotein lipase.6
Finally, arteriolosclerosis describes a variable group of lesions in arterioles which may be predominantly hyaline or hyperplastic, both of which are initiated by endothelial damage. Hyalinosis or hypertrophic hyalinization is characterized by brightly eosinophilic, amorphous material which expands vessel walls, and results from leakage of plasma proteins. In domestic animals, hyalinosis is most frequently seen in older dogs, and pigs within splenic arterioles. Additionally, in dogs, hyaline deposition may affect the intramural coronary arteries, meningeal arteries, and cerebral arteries. In the heart, it can result in multifocal intramural myocardial infarction (MIMI) which leads to congestive heart failure when paired with valvular endocardiosis (commonly found in older dogs). In pigs, pathologic changes associated with hyaline arteriolar deposits occurs with organomercurial poisoning (meninges), edema disease (gastric and colonic submucosa and cerebellar folia), and hepatosis dietetica and mulberry heart disease (heart). Hyperplastic arteriolosclerosis, is characterized by intimal changes (smooth muscle proliferation and concentric fibrosis, AKA “onion skinning”) with fibrinoid necrosis of the tunica media.6
In humans, the most common cause of arteriolosclerosis is systemic hypertension which can occur via primary or secondary means. In domestic animals, the most common cause of systemic hypertension is primary renal disease which is associated with primary or secondary hypertension. Primary systemic hypertension leads to decreased renal perfusion, subsequent activation of the renin-angiotensin-aldosterone system, and additional hypertension. Conversely, chronic renal disease can result in secondary hypertension by poor excretion of sodium and water and increased blood volume. One important and dangerous feature of hypertension is that it is self-perpetuating and can result in the death of the animal. The causes of hypertension and main clinical signs are described above by the contributor.6
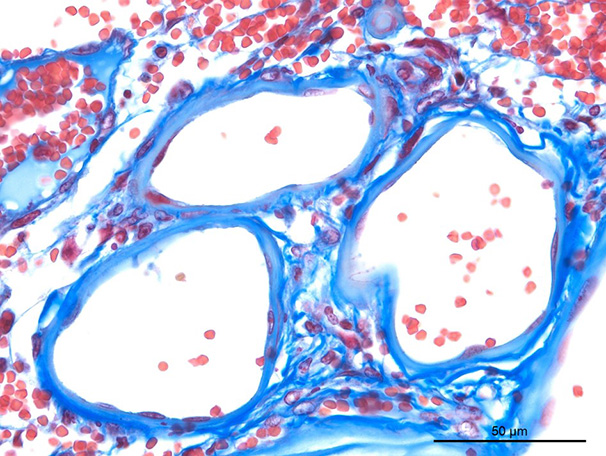
In this case, side-by-side viewing of the affected arterioles on HE and with a Masson’s trichrome deomonstrated the marked fibrosis of the vessel wall, which appeared as simple hyaline change on HE. The Masson’s trichrome identified abundant collagen (type I or III) that has effaced the tunica intima and media, smooth muscle cells. Additionally the Masson’s trichome helped to demonstrate the perivascular whorling of fibrocytes around the adventitia of smaller arterioles, in an attempt to stabilize them against the systemic hypertension.
Contributing Institution:
Colorado State University
http://csu-cvmbs.colostate.edu/academics/mip/Pages/default.aspx
References:
- Brown CA, Munday JS, Mathur S, Brown SA: Hypertensive Encephalopathy in Cats with Reduced Renal Function. Vet Pathol. 2005;42(5):642-649.
- Fishbein MC, Fishbein GA. Arteriosclerosis: facts and fancy. Cardiovasc Pathol. 2015;24(6):335-342.
- Maggio F, DeFrancesco TC, Atkins CE, Pizzirani S, Gilfer BC, Davidson MG: Ocular lesions associated with systemic hypertension in cats: 69 cases (1985-1998). JAVMA. 2000;217(5):695-702
- Maxie MG, Youssef S: Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed., vol. 1. Elsevier Science Direct E-Books. 2007: 335–343.
- Maxie MG, Robinson WF: Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th ed., vol 3. Elsevier Science Direct E-Books. 2007: 56-93
- Robinson WF, Robinson NA. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 3. St. Louis, MO: Elsevier; 2016:56-60.
- Suzuki K, Masawa M, Takatama M. The pathogenesis of cerebrovascular lesions in hypertensive rats. Med Electron Microsc. 2001; 34(4):230-9.