WSC 2022-2023:
Conference 16:
Case IV:
Signalment:
A 23.5-year-old female African green monkey (Chlorocebus aethiops sabaeus).
History:
This monkey had a three-year history gradual weight loss and a slow growing cranial abdominal mass.
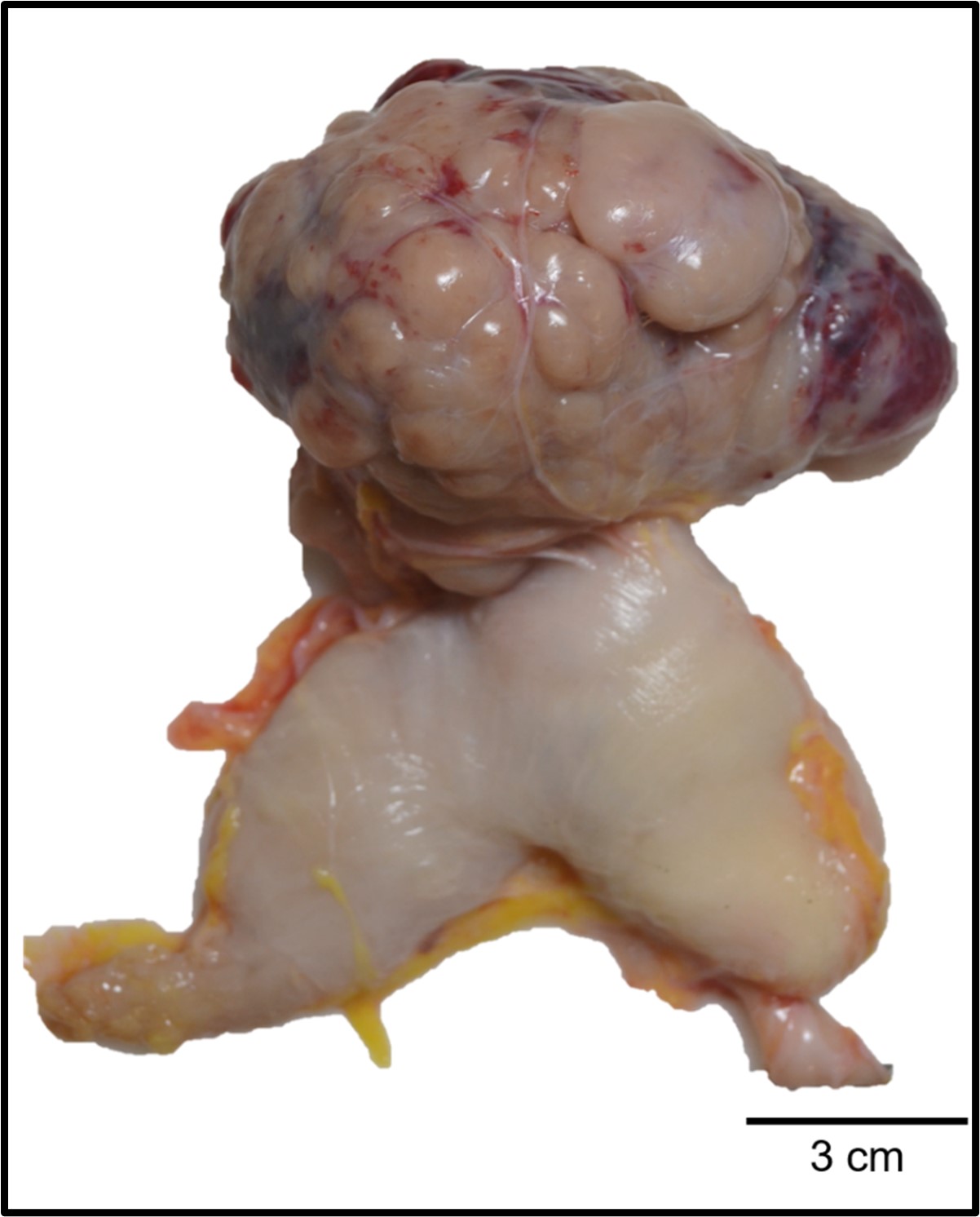
Gross Pathology:
A 12x8x7 cm mottled tan to red soft multilobular mass extended from the wall of the greater curvature of the body of the stomach into the peritoneum. The omentum was firmly adhered to the mass and the adjacent small intestines, stomach, liver, and left kidney. On sectioned surface, the mass was mottled tan to red with large red to black areas of necrosis.
Laboratory Results:
No laboratory results reported.
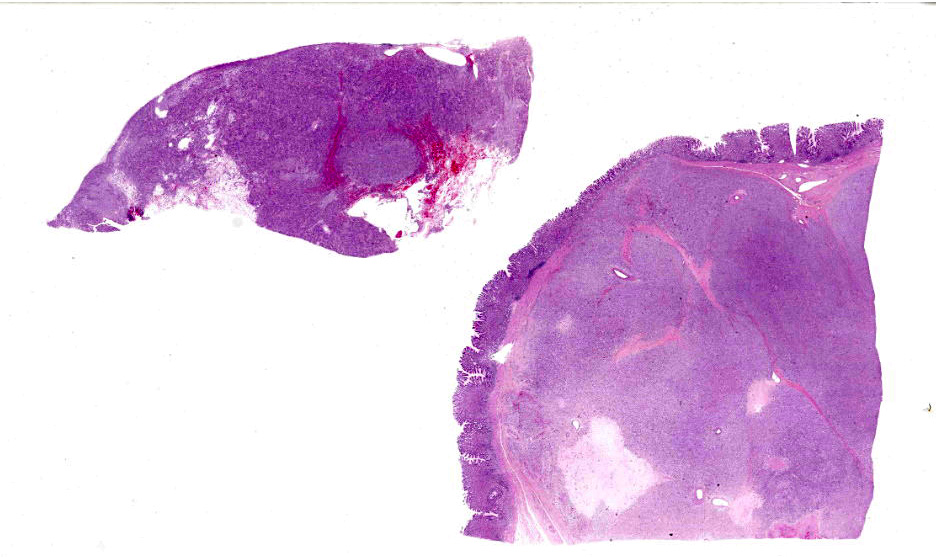
Microscopic Description:
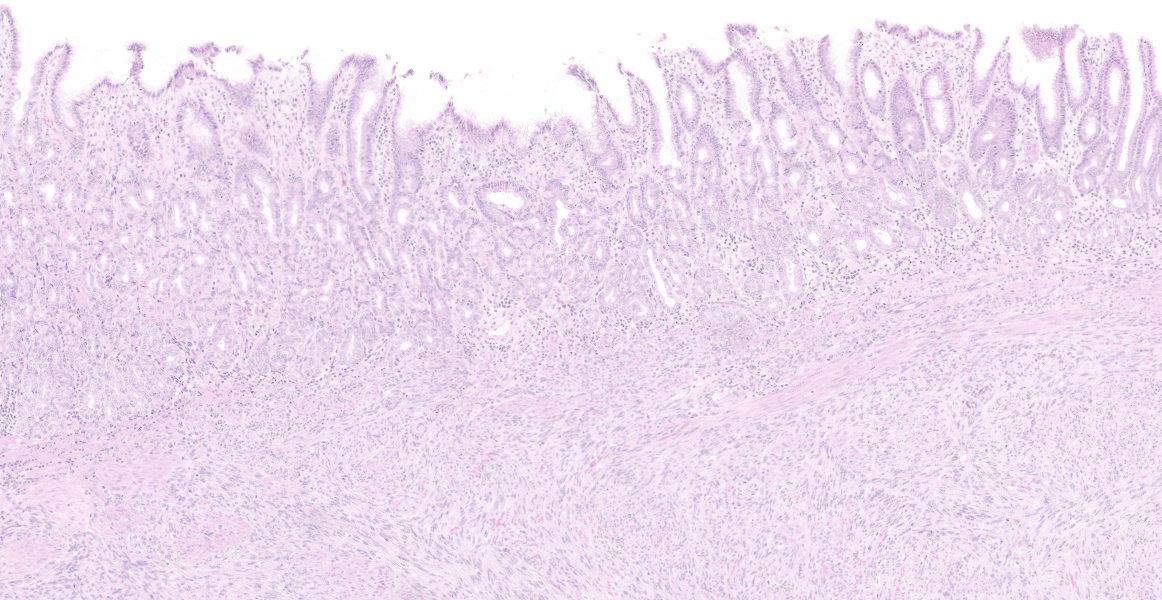
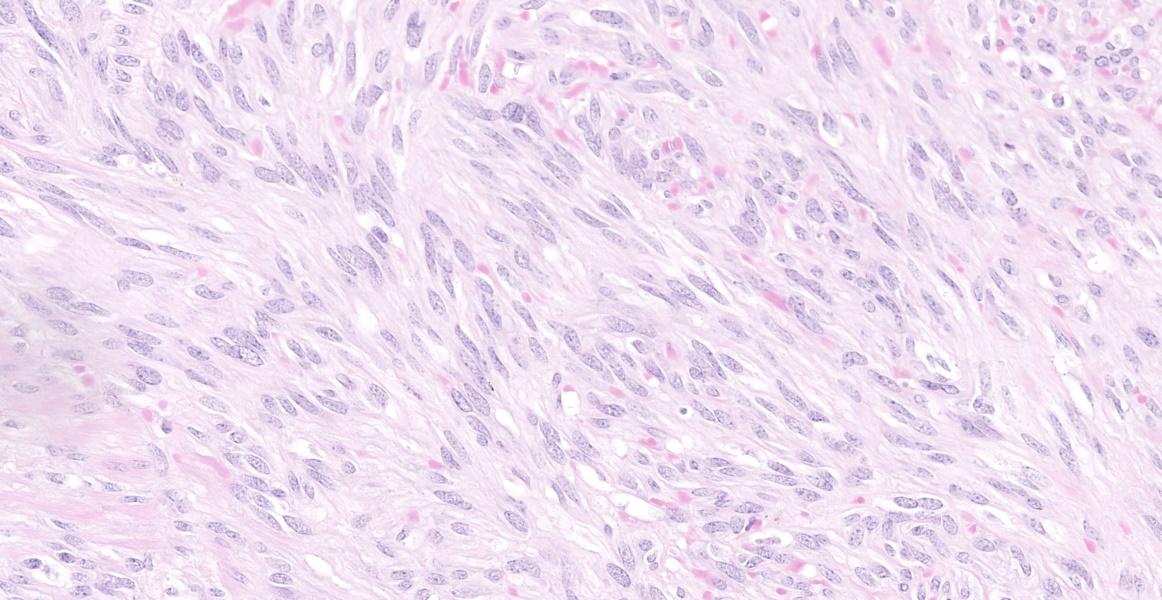
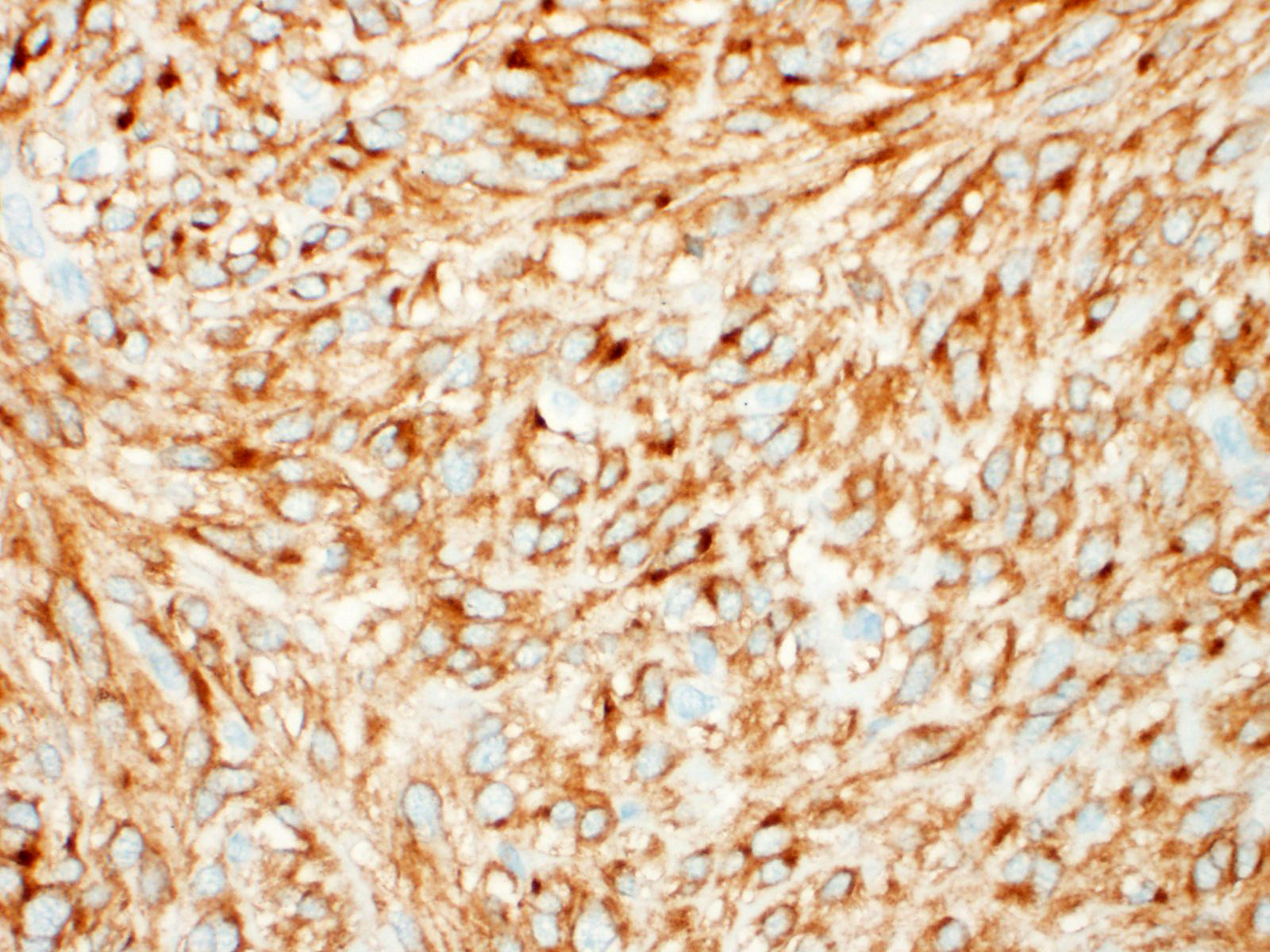
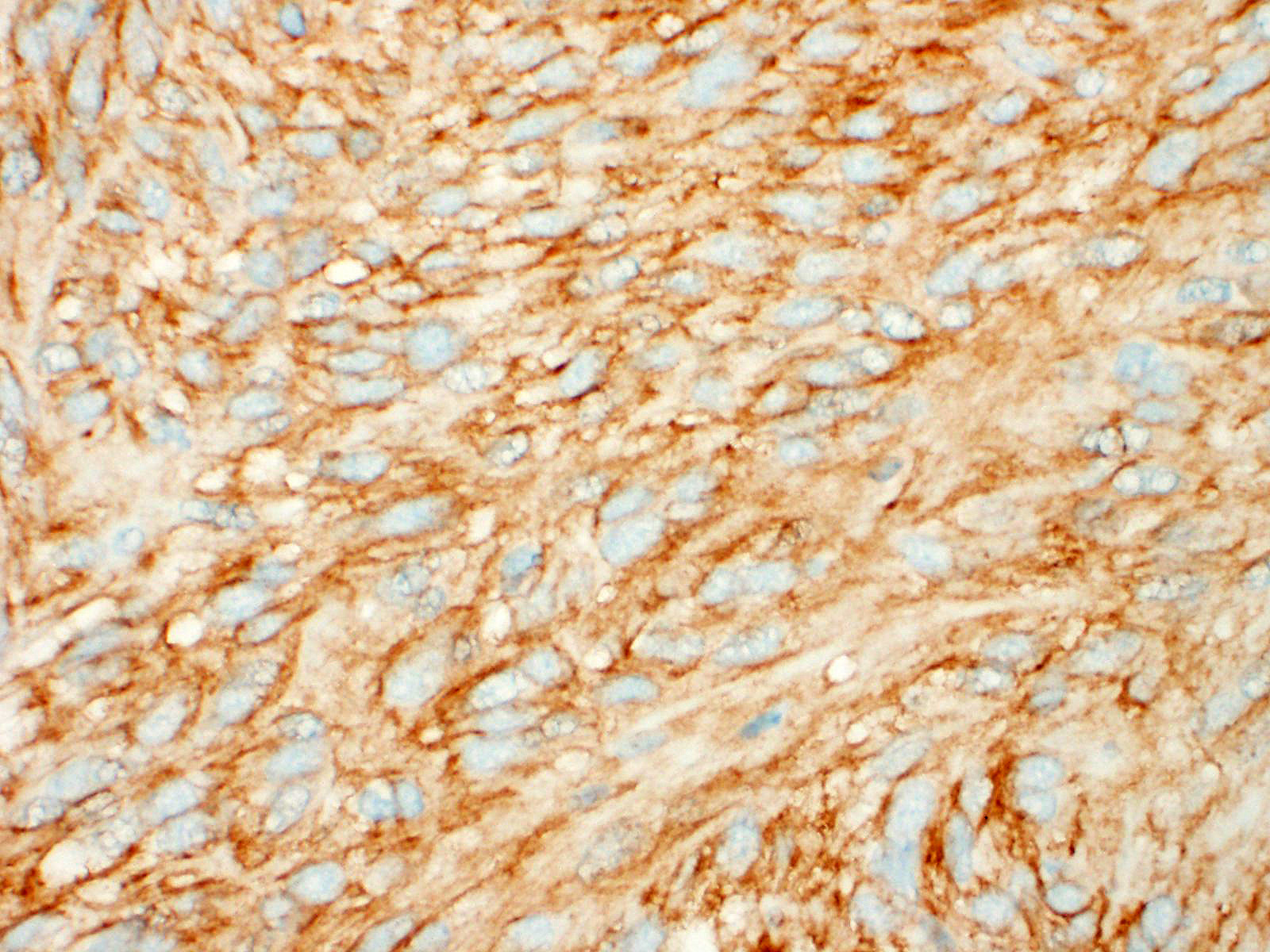
A well demarcated, partially encapsulated multilobular mass, expanding the muscularis externa and serosa, is composed of streams and bundles of closely spaced neoplastic mesenchymal cells supported by a fine fibrovascular stroma, and infiltrated by a few small aggregates of lymphocytes. The neoplastic cells range from 30x10-50x15 μm, have indistinct cell borders, abundant fibrillar to clear eosinophilic cytoplasm, and central oval to fusiform 10x10 -25x12 μm nuclei with finely stippled chromatin, without nucleoli. Mitoses occur at 0-1 per 10, 40x fields. Macrophages with intracytoplasmic golden-tan pigment (hemosiderin) infiltrate the capsule of the neoplasm. The overlying gastric mucosa is disorganized, thickened up to twice normal, with loss of polarity in the gastric pits and glands. Many of the gastric glands are lined by or have prominent parietal cells, and mitoses are numerous. Small hemorrhages are scattered throughout the lamina propria, and lymphoid aggregates along the deep mucosa are prominent. Few lymphocytes, eosinophils, and neutrophils infiltrate the muscularis mucosa. The smooth muscle of the tunica media of some arterioles in the submucosa are thickened, and the lumina are reduced in size. The neoplastic cells were immunohistochemically negative for smooth muscle actin (SMA) and positive for CD117.
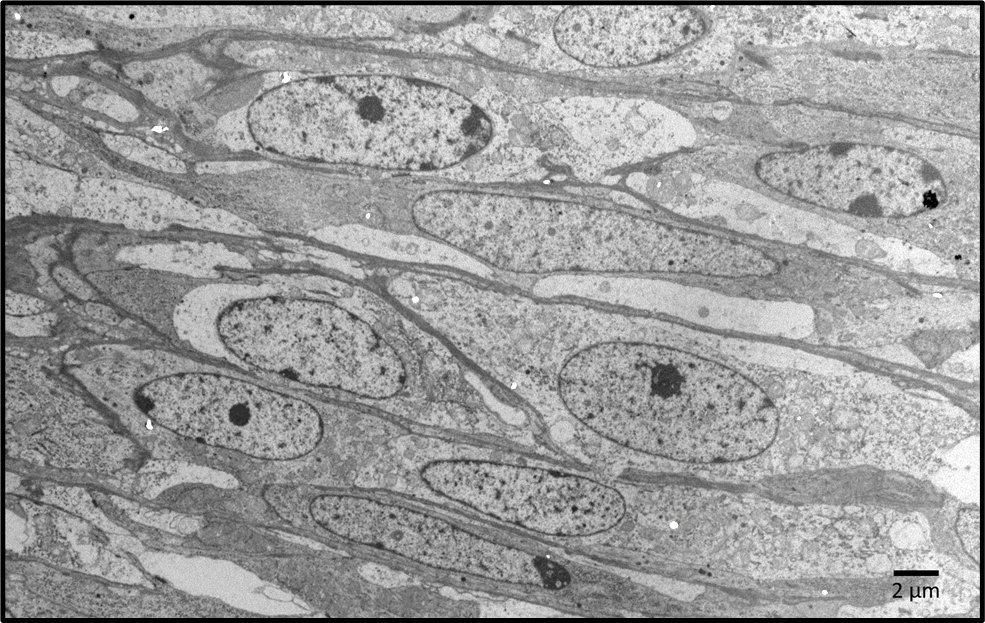
Transmission electron microscopy findings: The elongate neoplastic cells have medium electron-dense to electron lucent cytoplasm with few mitochondria, and fusiform euchromatic nuclei, some with single nucleoli. A few cells have two nuclei. The cells are separated by extracellular matrix containing 20 nm wide electron-dense fibrils, electron lucent spaces, and amorphous medium electron dense-material.
Contributor’s Morphologic Diagnoses:
Gastric body: Gastrointestinal stromal tumor
Contributor’s Comment:
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors of the gastrointestinal tract which are thought to originate from the interstitial cells of Cajal. They have been reported in rhesus macaques, chimpanzees, baboons, and spider monkeys, numerous domestic animals, and humans.1,2,3,10,13,14 In companion animals they most frequently arise in the intestine, whereas in humans and nonhuman primates, they primarily occur in the gastric cardia and body.1,2,13
Characteristic histologic findings include a herringbone pattern of mesenchymal cell proliferation supported by dense fibrovascular stroma, and in larger tumors necrosis is common. These tumors typically arise from the mucosa or submucosa, and transmural invasion is common.10 The majority of GISTs are CD117 (c-KIT), CD34, and DOG-1 (discovered on gastrointestinal stromal tumors protein-1) positive.13
The majority of GISTs contain a mutation in the KIT receptor tyrosine kinase gene, resulting in the overexpression of the KIT protein, which can be identified immunohistochemically with CD117 (c-KIT).12 CD34, an antigen for hematopoietic progenitor and endothelial cells is positive in some interstitial cells of Cajal and in the majority of GISTs in humans and other species.4,15 In humans and dogs, DOG-1 is a more sensitive and specific marker for GISTs than CD117. DOG-1 is independent of mutations in CD117, and has been documented to stain CD117- and CD34- negative GISTs.5,8
Ultrastructurally, GISTs are composed of primitive mesenchymal cells which may have non-membrane-bound electron lucent regions in the cytoplasm. Dense bodies, which are characteristic of smooth muscle, are present in leiomyomas and leiomyosarcomas, and absent in GISTs.6 Some GISTs, particularly in the intestinal tract and rectum in humans, may have skeinoid fibers, of which the composition is unknown. Histologically they appear as extracellular hyaline globules. Ultrastructurally, skeinoid fibers are composed of medium electron-dense fibrils resembling collagen but with a periodicity of ~43 nm.11,14 Skeinoid fibers were not identified in this case.
GISTs should be differentiated from leiomyomas, leiomyosarcomas, peripheral nerve sheath tumors, schwannomas, and neurofibromas because of the differences in metastatic potential and prognoses (Table 1). In humans, GISTs often arise in the gastric cardia and body, whereas leiomyomas are more common in the esophagus.9,17
|
Table 1 |
|
|
Differential Diagnoses |
Positive Immunohistochemical Stains |
|
Leiomyoma |
Smooth muscle actin (SMA) |
|
Leiomyosarcoma |
SMA |
|
Peripheral nerve sheath tumor |
CD99 / S100 |
|
Schwannoma |
S100 |
|
Neurofibroma |
S100 / CD34+ (focal) |
Contributing Institution:
Wake Forest School of Medicine
Department of Pathology, Section on Comparative Medicine
Medical Center Boulevard, Winston-Salem, NC 27157
www.wakehealth.edu
JPC Diagnosis:
Stomach: Gastrointestinal stromal tumor.
JPC Comment:
As the contributor mentions, one of the differentials for gastrointestinal stromal tumors (GISTs) is a smooth muscle neoplasm, such as leiomyoma and leiomyosarcoma.7 Historically, human GISTs humans were diagnosed as smooth muscle neoplasms prior to ultrastructural evaluation and the discovery of the CKIT protooncogene marker of these neoplasms.7 Leiomyomas and leiomyosarcomas have historically been the most common spindle cell neoplasm of the gastrointestinal tract in rats and mice in the National Toxicology Program’s two-year bioassays, so researchers recently conducted a retrospective study evaluating CKIT expression in smooth muscle tumors of these species.7 In B6C3F1/N mice, 22 of 32 tumors (69%) previously diagnosed as leiomyoma/leiomyosarcoma were CKIT positive, indicating they are likely GISTs.7 Most (16) of these tumors arose in the cecum, with fewer in the colon and stomach. In rats, all of the tumors were negative for CKIT, and most were positive for desmin and smooth muscle actin, confirming their diagnosis as smooth muscle tumors.7 These surprising results indicate that GISTs are more common than leiomyoma/leiomyosarcomas in mice in the NTP bioassays.7
Another recent study described gastrointestinal stromal tumors (GISTs) in four guinea pigs of advanced age.16 Three animals had neoplasms in the gastric wall at the cardia, while the fourth was in the small intestine.16 One of the gastric GISTs metastasized to the duodenal and cecal mesentery.16 The gastric GISTs were positive for c-KIT, DOG-1, and smooth muscle actin.16 The small intestinal GIST was negative for smooth muscle actin, but positive for c-KIT and DOG-1.16 In all neoplasms, atypia was mild and mitotic figures were rare. Additionally, all cases had deletion mutations in exon 11 of the Kit gene.16
References:
- Banerjee M, Lowenstine LJ, Munn RJ. Gastric stromal tumors in two rhesus macaques (Macaca mulatta). Vet Pathol. 1991; 28: 30-36.
- Bommineni YR, Dick EJ, Jr., Hubbard GB. Gastrointestinal stromal tumors in a baboon, a spider monkey, and a chimpanzee and a review of the literature. J Med Primatol. 2009; 38: 199-203.
- Brown SL, Anderson DC, Dick EJ, Jr., Guardado-Mendoza R, Garcia AP, Hubbard GB. Neoplasia in the chimpanzee (Pan). J Med Primatol. 2009; 38: 137-144.
- Bure I, Braun A, Kayser C et al. The expression of hematopoietic progenitor cell antigen CD34 is regulated by DNA methylation in a site-dependent manner in gastrointestinal stromal tumours. International Journal of Cancer 141: 2296-2304, 2017
- Dailey DD, Ehrhart EJ, Duval DL, Bass T, Powers BE. DOG1 is a sensitive and specific immunohistochemical marker for diagnosis of canine gastrointestinal stromal tumors. J Vet Diagn Invest. 2015; 27: 268-277.
- Ferenczy A, Richart R, Okagaki T. A comparative ultrastructural study of leiomyosarcoma, cellular leiomyoma, and leiomyoma of the uterus. Cancer. 1971; 28: 1004-1018.
- Janardhan KS, Venkannagari P, Jensen H, et al. Do GISTs Occur in Rats and Mice? Immunohistochemical Characterization of Gastrointestinal Tumors diagnosed as Smooth Muscle Tumors in the National Toxicology Program. Toxicol Pathol. 2019; 47(5):577-584.
- Jung JH, Im S, Choi HJ, Lee YS, Jung ES. Gastrointestinal stromal tumor with dedifferentiation to undifferentiated pleomorphic sarcoma. Pathol Int. 2013; 63: 479-482.
- Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003; 23: 283-304, 456; quiz 532.
- Meuten DJ. Tumors in Domestic Animals. Fifth edition. Ames, IO: Wiley Blackwell. 2017.
- Min KW: Gastrointestinal stromal tumor: an ultrastructural investigation on regional differences with considerations on their histogenesis. Ultrastruct Pathol. 2010; 34: 174-188.
- Novelli M, Rossi S, Rodriguez-Justo M, Taniere P et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010; 57: 259-270.
- Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019; 10: 144-154.
- Saito T, Ueno M, Ota Y et al. Histopathological and clinical characteristics of duodenal gastrointestinal stromal tumors as predictors of malignancy. World J Surg Onc. 2013; 11.
- Saturday GA, Lasota J, Frost D, Brasky KB, Hubbard G, Miettinen M: KIT-positive gastrointestinal stromal tumor in a 22-year-old male chimpanzee (Pan troglodites). Vet Pathol. 2005; 42: 362-365.
- Ueda K, Takanosu M, Kagawa Y, et al. Gastrointestinal stromal tumors with Kit gene mutation in 4 guinea pigs (Cavia porcellus). Vet Pathol. 2022. 59(5): 740-746.
- Yang DY, Wang X, Yuan WJ, Chen ZH: Metastatic pattern and prognosis of gastrointestinal stromal tumor (GIST): a SEER-based analysis. Clin Transl Oncol. 2019.