Signalment:
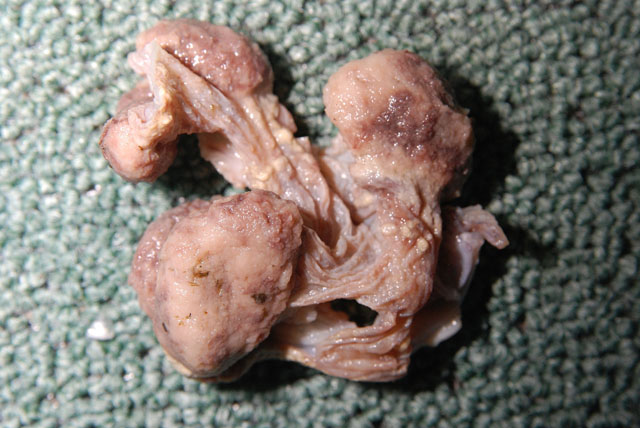
Gross Description:
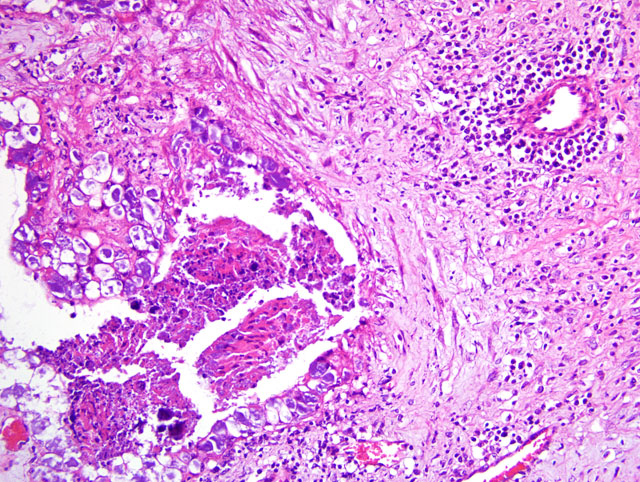
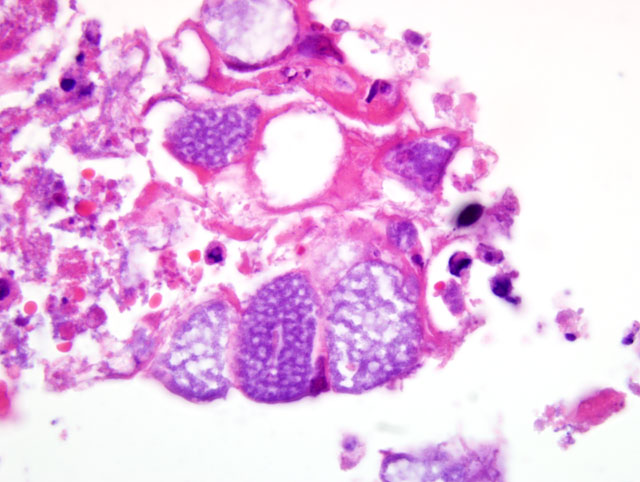
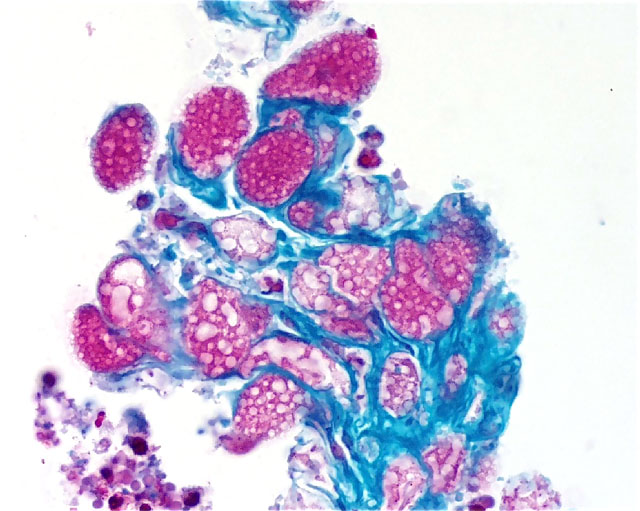
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Culture: No Brucella sp. or other significant bacteria from the placenta or fetus (4+ alpha Streptococcus sp. and 4+ E. coli in the abomasal fluid and placenta). Leptospira F.A.: Negative on fetal kidneys. Chlamydia (Chlamydophila) PCR: Negative. Coxiella burnetti immunohistochemistry: Positive (done at UC Davis).
Condition:
Contributor Comment:
Coxiella, Brucella, and Chlamydophila infections in sheep and goats cause both cotyledonary and intercotyledonary necrosis (3,4,8) and all 3 agents target the intracellular fetal trophoblast cells.(10) Campylobacter infection causes similar lesions with necrotic inflammation also being in the fetal tissues.(4,5) Campylobacteriosis only affects the cotyledons in cows.(3)
One survey in France (8) found that infected or carrier cows and goats generally shed Coxiella in their milk and sheep mostly in vaginal secretions and feces. Since human infection is generally by aerosol, this might explain why humans are at greater risk from contact with sheep. This study did not find a correlation between recent parturition and shedding of organisms.
A PCR survey (5) of milk goat abortions on the island of Sardinia found 82.7% negative for an infectious agent and 16.3% positive with the results as below from 23 caprine fetuses and 8 placentas:
- Toxoplasma: 13% / 25% (fetus / placenta)
- Salmonella abortusovis: 0% / 0%
- C. burnetti: 0% / 12.5%
- Chlamydophila abortus: 0% / 12.5%
- Neospora caninum: 8.6% / 0%
Goats may abort with Coxiella infection in 2 consecutive pregnancies although they have antibodies, so there may not be protective immunity after the first infection and a carrier state is possible.(1) Another survey found 9% of the goat abortions being due to Coxiella infection, but lesions were usually limited to the placenta and many submissions did not have the placenta.(7)
JPC Diagnosis:
Conference Comment:
While both organisms can cause both cotyledonary and intercotyledonary necrosis, the gross lesions of Chlamydophila infection affect the cotlyledons and intercotyledonary regions in roughly equal proportions, while the placental lesions in Coxiella burnetti infection are most prominent in the intercotyledonary region. Both organisms can cause vasculitis in the placenta, although it is characteristically more marked in Chlamydophila abortion.(4)
References:
2. Brogden KA: Cytopathology of pathogenic prokaryotes. In: Ultrastructural pathology: the comparative cellular basis of disease, ed. Cheville NF, 2nd ed., p. 476, Wiley-Blackwell, Ames, IA, 2009
3. Buergelt CL: Color Atlas of Reproductive Pathology of Domestic Animals. Mosby, St. Louis, MO, pp. 179-187, 1997
4. Kennedy PC, Miller RB: The female genital system. In: Pathology of Domestic Animals, eds. Jubb KVF, Kennedy PC, Palmer N, 4th ed., vol. 3, pp. 396-419, Academic Press, San Diego, CA, 1993
5. Ladds PW, We F-M, Chang W-F, Shyu J-J: A Color Atlas of Veterinary Reproductive Pathology. Pig Research Institute, Taiwan, pp. 292-294, 297-298, 1997
6. Masala G, Porcu R, Daga C, Denti S: Detection of pathogens in ovine and caprine samples from Sardinia, Italy, by PCR. J Vet Diagn Invest 19:96-98, 2007
7. Moeller RB: Causes of caprine abortion: diagnostic assessment of 211 cases (1991-2998), J Vet Diag Invest 13:265-270, 2001
8. Moore JD, Barr BC, Daft BM, OConnor MT: Pathology and diagnosis of Coxiella burnetti infection in a goat herd. Vet Pathol 28:81-84, 1991
9. Rodolakis A, Berri M, H+�-�chard C, Caudron C, et al: Comparison of Coxiella burnetti infection in milk of dairy bovine, caprine, and ovine herds. J Dairy Sci 90:5352-5360, 2007
10. S+�-�nchez J, Souriau A, Buend+�-�a AJ, et al: Experimental Coxiella burnetti infection in pregnant goats: a histopathological and immunohistochemical study. J Comp Path 135:108-115, 2006