CASE II:
Signalment:
Two 6-month-old, black, American mink (Neovison vison), one male and one female
History:
A farm of approximately 50,000 all black, American mink experienced increased mortality within two shelters. Approximately 15-20 animals died over the course of two days, with two submitted for necropsy.
Gross Pathology:
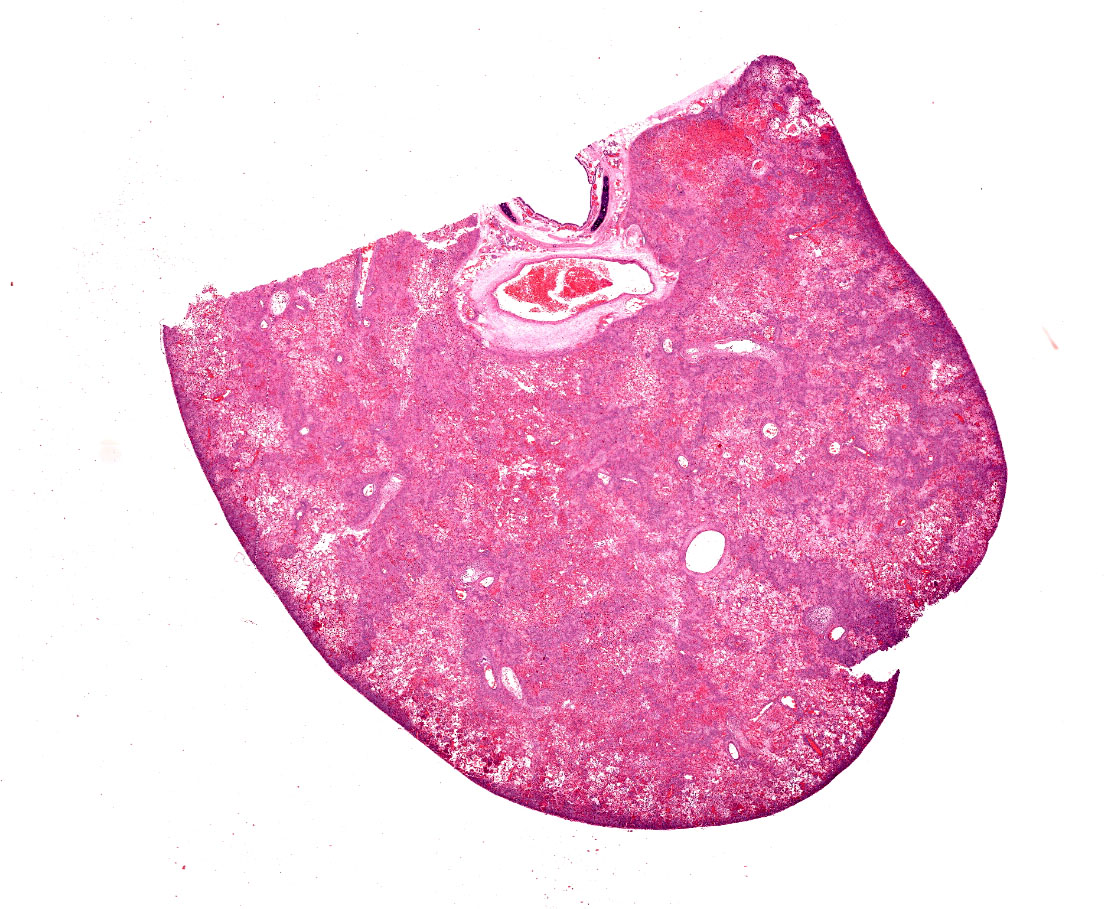
Two reportedly six-month-old, black mink weighing 1.9kg (female) and 2.4kg (male) were necropsied. The bodies of both were in good condition (BCS 3/5) and postmortem autolysis was moderate. The lungs in both animals were diffusely firm and mottled bright red to purple with areas of paler color. At least one section of lung from both animals sank in formalin; other sections floated.
Laboratory Results:
Black Mink, Female, Lung Tissue, Aerobic culture: 4+ Pseudomonas aeruginosa.
Black Mink, Male, Lung Tissue, Aerobic culture: 4+ Pseudomonas aeruginosa - sensitivity performed on female, see Table 3-1.
|
Table 3-1 Antibiotic |
MIC (µg/ml) |
Interpretation |
|
Amikacin |
< 1.000 µg/ml |
Sensitive |
|
Amoxicillin/Clavulanic Acid |
>64.000 µg/ml |
Resistant |
|
Ampicillin |
>48.000 µg/ml |
Resistant |
|
Cefpodoxime |
>64.000 µg/ml |
Resistant |
|
Cephalothin |
>128.000 µg/ml |
Resistant |
|
Chloramphenicol |
32.0 µg/ml |
Intermediate |
|
Clindamycin |
>12.000 µg/ml |
Resistant |
|
Enrofloxacin |
0.4 µg/ml |
Sensitive |
|
Gentamicin |
1.5 µg/ml |
Sensitive |
|
Imipenem |
< 0.750 µg/ml |
Sensitive |
|
Marbofloxacin |
|
Sensitive |
|
Orbifloxacin |
2.0 µg/ml |
Intermediate |
|
Tetracycline |
16.0 µg/ml |
Resistant |
|
TMP/Sulfa |
>12.000 µg/ml |
Resistant |
|
Tobramycin |
< 0.380 µg/ml |
Sensitive |
Microscopic Description:
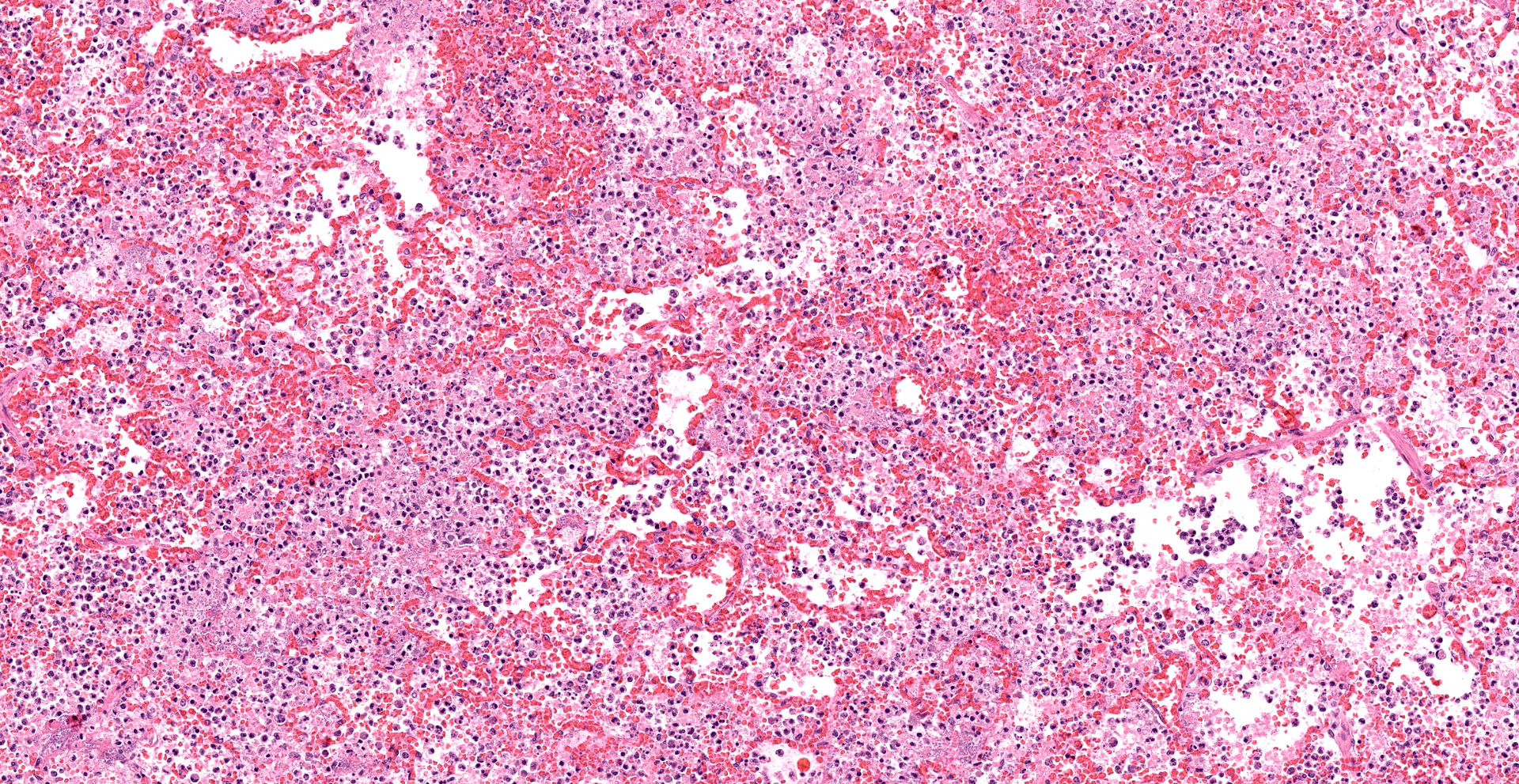
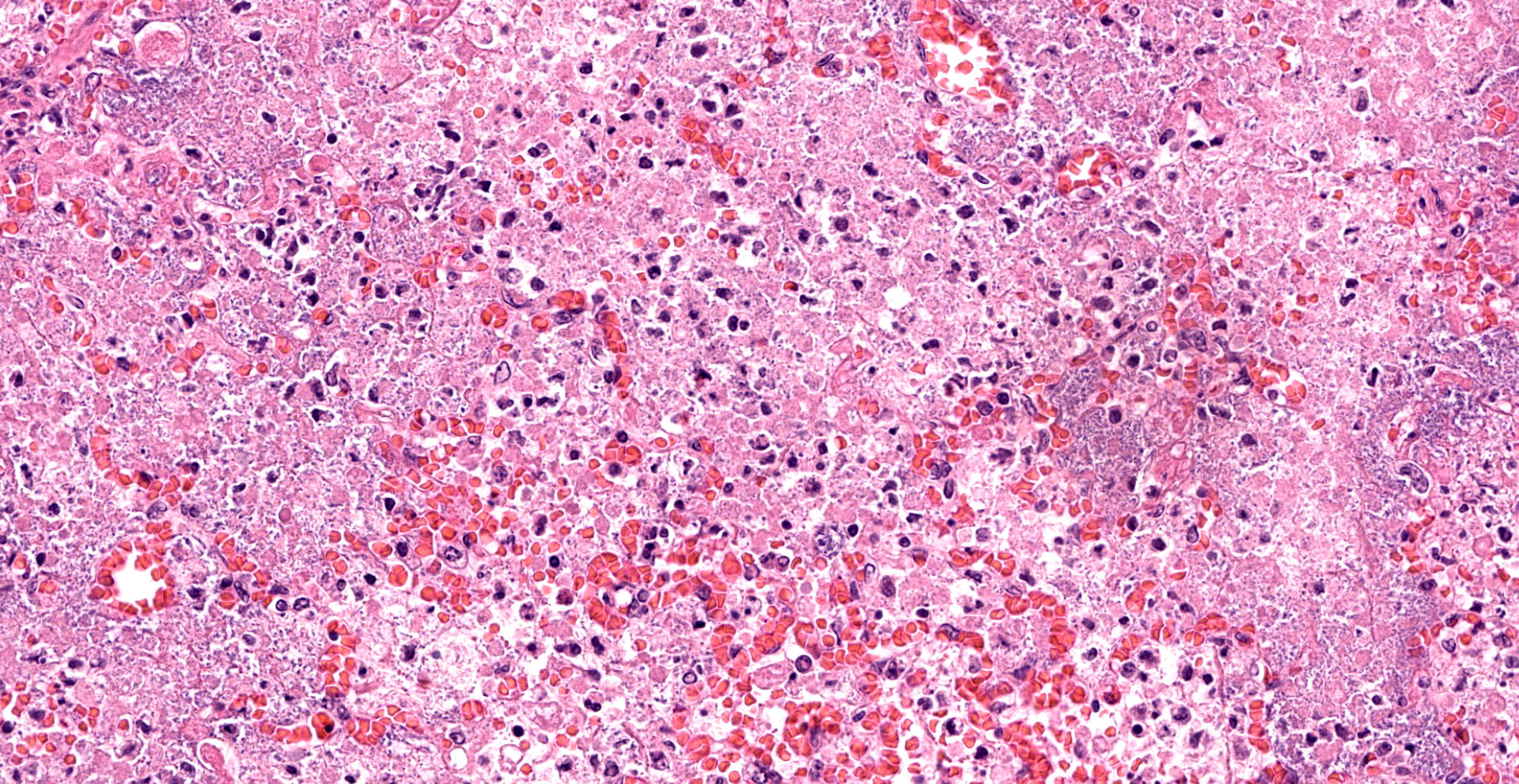
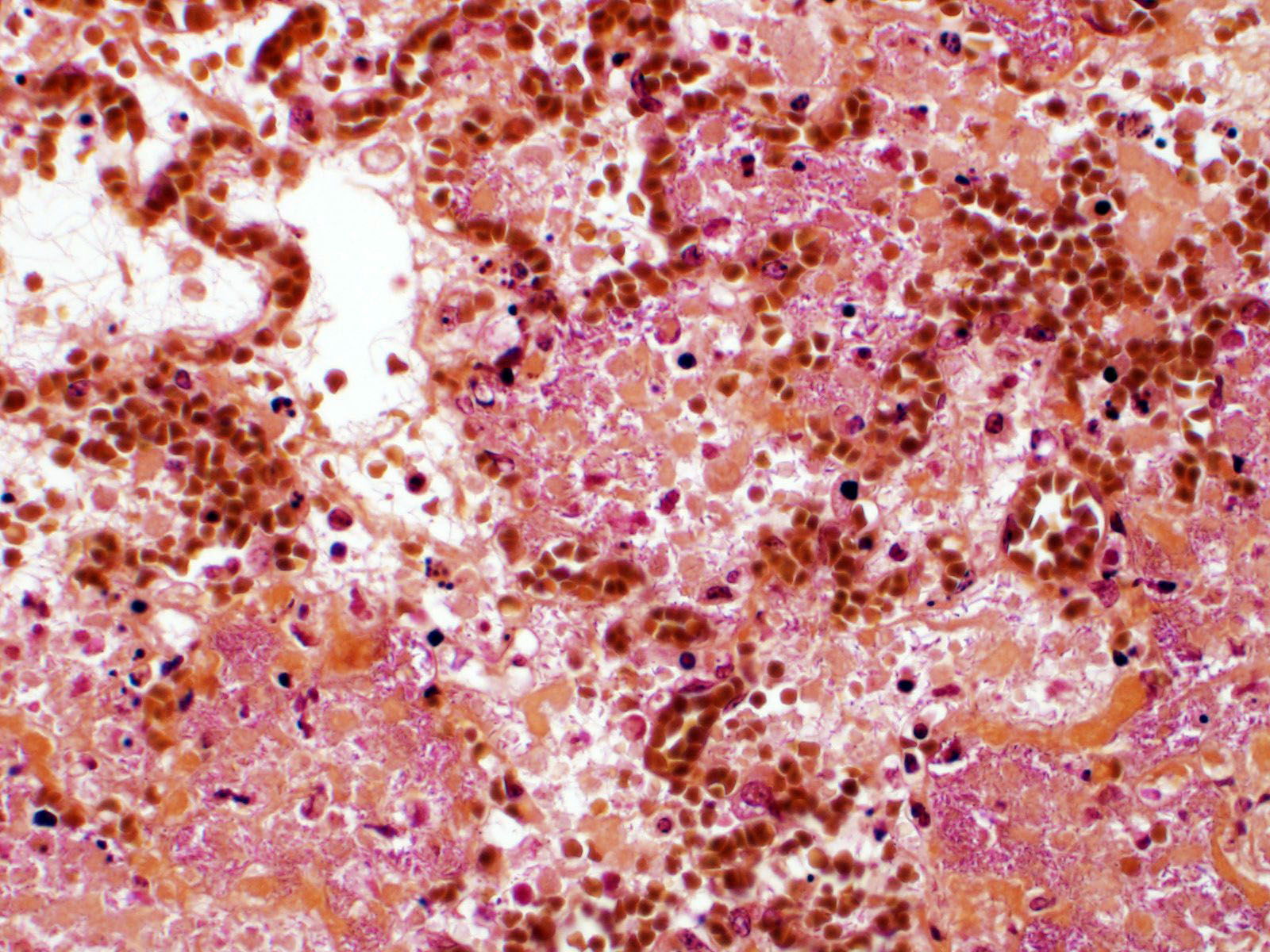
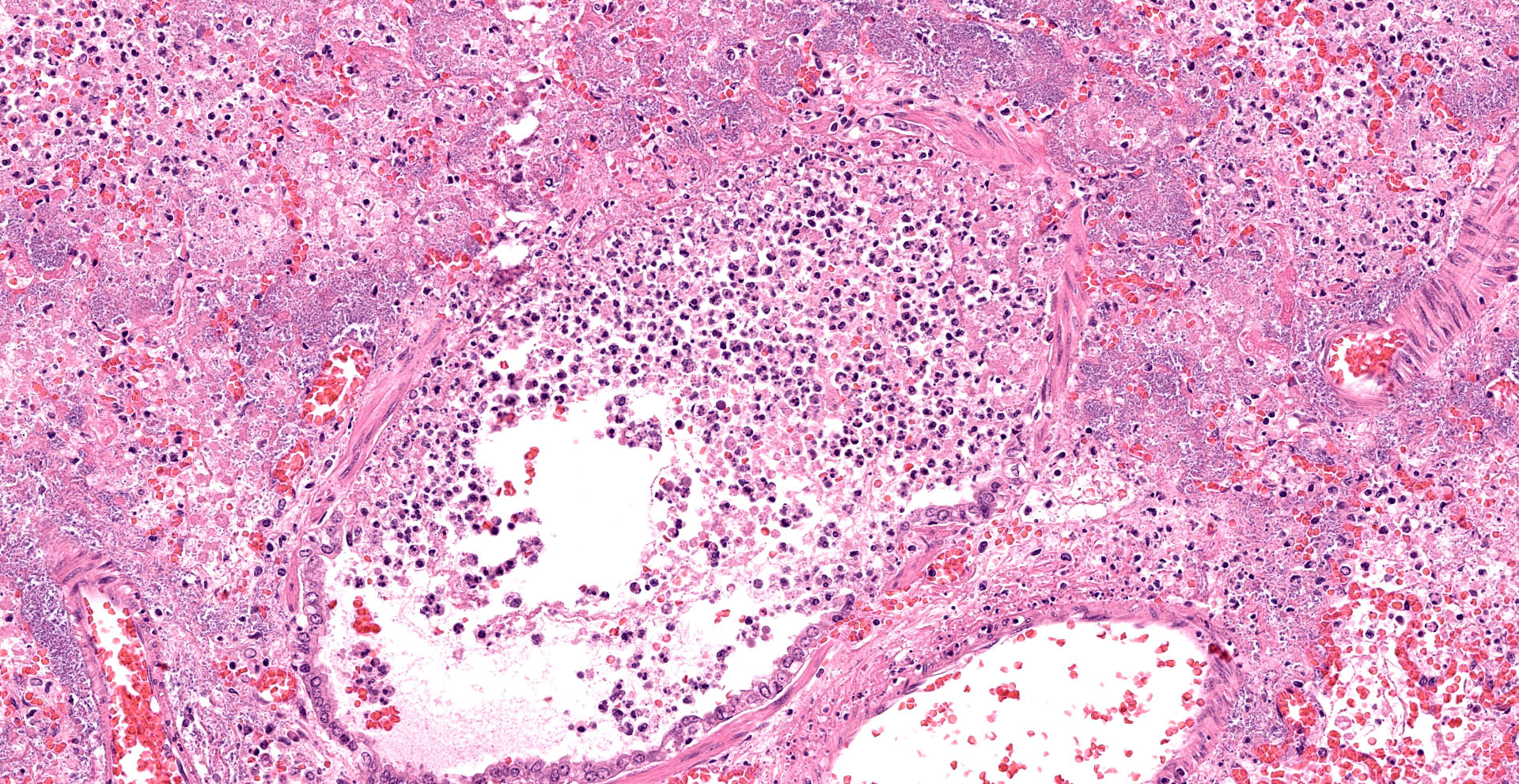
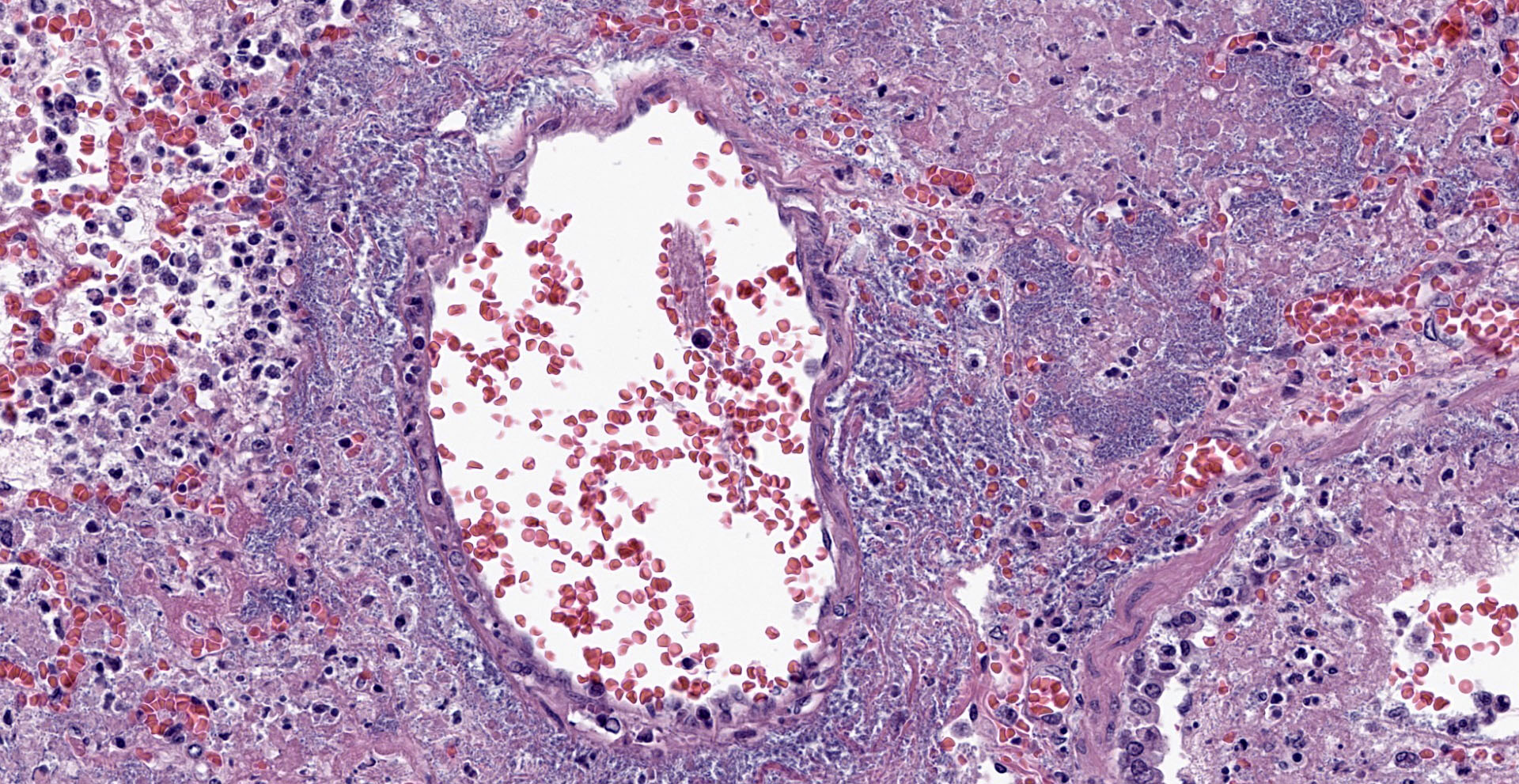
Lung. Sections of lung in which there is diffuse, submassive, coagulative necrosis and suppurative inflammation effacing up to 90% of the pulmonary parenchyma. These areas contain necrotic cell debris, seroproteinaceous fluid, neutrophils and myriad clusters of small, Gram-negative bacteria. In nearby alveoli, there is intraalveolar hemorrhage. Several bronchioles are occluded or partially occluded by aggregates of fibrin and neutrophils, and cell debris.
Contributor?s Morphologic Diagnoses:
Lung: Bronchopneumonia, severe, acute, diffuse, hemorrhagic, fibrinosuppurative.
Contributor?s Comment:
Pseudomonas aeruginosa is a known cause of contagious, hemorrhagic bronchopneumonia in farmed mink, although most mustelids are susceptible.3 It was first described in 1953.9 It is a seasonal disease most commonly seen in September to November. It is often acute and fatal and some mink can be found with blood around the nostril and mouth without previous signs of illness.16,17 Experimentally, the organism can be carried in the nasal cavity without causing disease. There are various serotypes and genotypes and many of these form biofilms.13, 18 Also, numerous vaccines and other therapeutic strategies have been investigated.7 In one study, Phage YH30 delivered intranasally reduced disease severity.6 Antibiotic resistance is common in Pseudomonas spp. and can vary geographically and temporally; antibiotic resistance and sensitivities seen here are different than those previously reported.13 Outbreaks of hemorrhagic pneumonia have been associated with hemolytic Escherichia coli infection as well.15
Contributing Institution:
Oregon State University, Carlson College of Veterinary Medicine, Veterinary Diagnostic Laboratory
https://vetmed.oregonstate.edu/diagnostic
JPC Diagnosis:
Lung: Pneumonia, fibrinosuppurative, diffuse, severe, with septal necrosis, vasculitis and innumerable perivascular and alveolar bacilli.
JPC Comment:
The distribution of histologic lesions led to spirited discussion among conference participants regarding the correct morphologic classification of this pneumonia. The pathogenesis of Pseudomonas pneumonia in mink is consistent with a bronchopneumonia, with aerogenous delivery of bacteria and inflammation targeting the epithelium of bronchi and bronchioles.11 In this case, however, much of the necrosis and bacterial colonies are centered on blood vessels, leading a few participants to consider embolic pneumonia as a morphologic pattern. Embolic pneumonias are caused by hematogenous showering of bacterial emboli from a nidus of infection resulting in multifocal lesions throughout all lung lobes.11 Possible sources of emboli include hepatic abscesses, omphalophlebitis, or endocarditis, and embolic pneumonia has been described secondary to Pseudomonas aeruginosa in a puppy with necrotizing enteritis.11 The contributor did not describe any possible nidus of infection in this case, and perivascular localization of bacteria is characteristic in Pseudomonas pneumonias of both mink and rats.15 Since the pathogenesis and histologic appearance do not fit into one precise category, participants decided not to further subtype the morphologic diagnosis beyond pneumonia.
Pseudomonas aeruginosa is typically an opportunistic pathogen which causes disease in immunocompromised animals or animals with surgical implants.10 The bacteria thrives in moist environments, such as water bottles in laboratory animal enclosures, and gains entry through the gastrointestinal tract or by penetrating inflamed or injured oronasal mucosa or intertriginous skin.1, 10 In immunocompromised mice and rats, infection can lead to bacteremia and Gram negative septicemia with vasculitis, thrombosis, and necrosis in multiple organs, including the lung, liver, spleen, and kidneys.1 The bacteria can also lead to deep pyoderma, septic arthritis, discospondylitis, otitis, and a number of other conditions.1, 5, 10 Due to the bacteria?s propensity for moist environments, improperly chlorinated pools and hot tubs can harbor P. aeruginosa, and human skin can be inoculated by abrasive surfaces, resulting in two self-limiting but painful conditions: acute folliculitis and pseudomonas hot-foot syndrome (nodules of suppurative inflammation on the hands and feet).4
The pathogenicity of P. aeruginosa is determined by a number of virulence factors, including exotoxins which are toxic to macrophages and epithelial cells, a type III secretion system for injecting proteins directly into cells, elastase which destroys pulmonary microbe clearance mechanisms like mucin, and immunomodulatory alkaline protease A.10,14 P. aeruginosa also produces two relatively unique virulence factors: pyocyanin, a redox metabolite, and pyoverdine, a siderophore.8 These virulence factors are pigments that can impart a blue-green discoloration to superficial infections.8 Rabbits with moist dermatitis and P. aeruginosa develop blue-green tinged fur.11 In humans, Pseudomonas can cause green nail and green foot syndromes, and, when associated with intertrigo, causes blue-green staining of undergarments.8 Indeed, when P. aeruginosa was first cultured from blue-green pus in superficial wounds in the 1880?s, it was aptly dubbed Bacillus pyocyaneus, or ?bacteria of blue pus?.18 The current name also references this distinctive hue: aerugo is Latin for copper rust, which is characteristically green.18
References:
- Barthold SW, Griffey SM, Percy DH. Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IO: John Wiley & Sons, Inc. 2016; 66, 143.
- Cole LK. Otitis Externa. In: Greene CE, ed. Infectious Disease of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier Saunder. 2012; 886-891.
- Fernandez-Moran J. Chapter 49: Mestelidae. Zoo and Wild Animal Medicine. Fowler ME and Miller RE, eds. Saunders: St. Louis, MO. 501-516, 2003.
- Fiorillo L, Zucker M, Sawyer D, Lin AN. The Pseudomonas Hot-Foot Syndrome. The New England Journal of Medicine. 2001; 354:335-338.
- Greene CE, Bennett D. Musculoskeletal Infections. In: Greene CE, ed. Infectious Disease of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier Saunder. 2012; 892-915.
- Gu J, Li X, Yang M, Du C, Cui Z, Gong P, Xia F, Song J, Zhang L, Li J, Yu C, Sun C, Feng X, Lei L, Han W. Therapeutic effect of Pseudomonas aeruginosa phage YH30 on mink hemorrhagic pneumonia. Vet Microbiol. 190:5-11, 2016.
- Homma JY, Abe C, Yanagawa R, Noda H. Effectiveness of immunization with multicomponent vaccines in protection against hemorrhagic pneumonia due to Pseudomonas aeruginosa infection in mink. Rev Infect Dis. 5 Suppl 5:S858-66, 1983.
- Kaya TI, Delialioglu N, Yazici AC, Tursen U, Ikizoglu G. Medical Pearl: Blue underpants sign - A diagnostic clue for Pseudomonas aeruginosa intertrigo of the groin. J Am Acad Dermatol. 2005; 53(5): 869-871.
- Knox B. Pseudomonas aeruginosa som årsag til enzootiske infektioner hos mink (Pseudomonas aeruginosa as the cause of enzootic infections in mink). Nord Vet Med. 5:731, 1953.
- Koenig Amie. Gram-Negative Bacterial Infections. In: Greene CE, ed. Infectious Disease of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier Saunder. 2012; 349-354.
- Lopez A, Martinson SA. Respiratory System, Thoracic Cavities, Mediastinum, and Pleura. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease.7th St. Louis, MO: Eslevier. 2022; 581-591.
- O?Donoghue PN, Whatley BF. Pseudomonas aeruginosa in Rabbit Fur. Laboratory Animals. 1971; 5:251-255.
- Qi J, Li L, Du Y, Wang S, Wang J, Luo Y, Che J, Lu J, Liu H, Hu G, Li J, Gong Y, Wang G, Hu M, Shiganyan, Liu Y. The identification, typing, and antimicrobial susceptibility of Pseudomonas aeruginosa isolated from mink with hemorrhagic pneumonia. Vet Microbiol 70(3-4):456-61, 2014.
- Qian Zhu, Hui Peng, Han Li et al. Serotypes and virulence genes of Pseudomonas aeruginosa isolated from mink and its pathogenicity in mink. Microbial Pathogenesis. 2020; 139:1-5.
- Salomonsen CM, Boye M, Høiby N, Jensen TH, Hammer AS: Comparison of histological lesions in mink with acute hemorrhagic pneumonia associated with Pseudomonas aeruginosa or Escherichia coli. Can J Vet 77(3):199-204, 2013.
- Salomonsen CM, Chriél M, Jensen TH, Rangstrup-Christensen L, Høiby N, Hammer AS. Effect of infectious dose and season on development of hemorrhagic pneumonia in mink caused by Pseudomonas aeruginosa. Can J Vet Res. Jul;77(3):221-5, 2013.
- Shimizu T, Homma JY, Aoyama T, Onoera T, Noda H. Virulence of Pseudomonas aeruginosa and spontaneous spread of Pseudomonas pneumonia in a mink ranch. Infect Immun.10:16-20, 1974.
- Villavicencio RT. The History of Blue Pus. J Am Coll Surg. 1998: 187(2): 212-216.
- Zhao Y, Guo L, Li J, Fang B, Huang X. Molecular epidemiology, antimicrobial susceptibility, and pulsed-field gel electrophoresis phenotyping of Pseudomonas aeruginosa isolates from mink. Can J Vet Res. 2018; 82(4): 256-263.