WSC 2022-2023
Conference 18
Case II:
Signalment:
One-month-old, male intact Grant’s gazelle (Nanger granti granti)
History:
Acute onset of lethargy, anorexia, and pyrexia (106 F?). After initial supportive care, euthanasia was elected.
Gross Pathology:
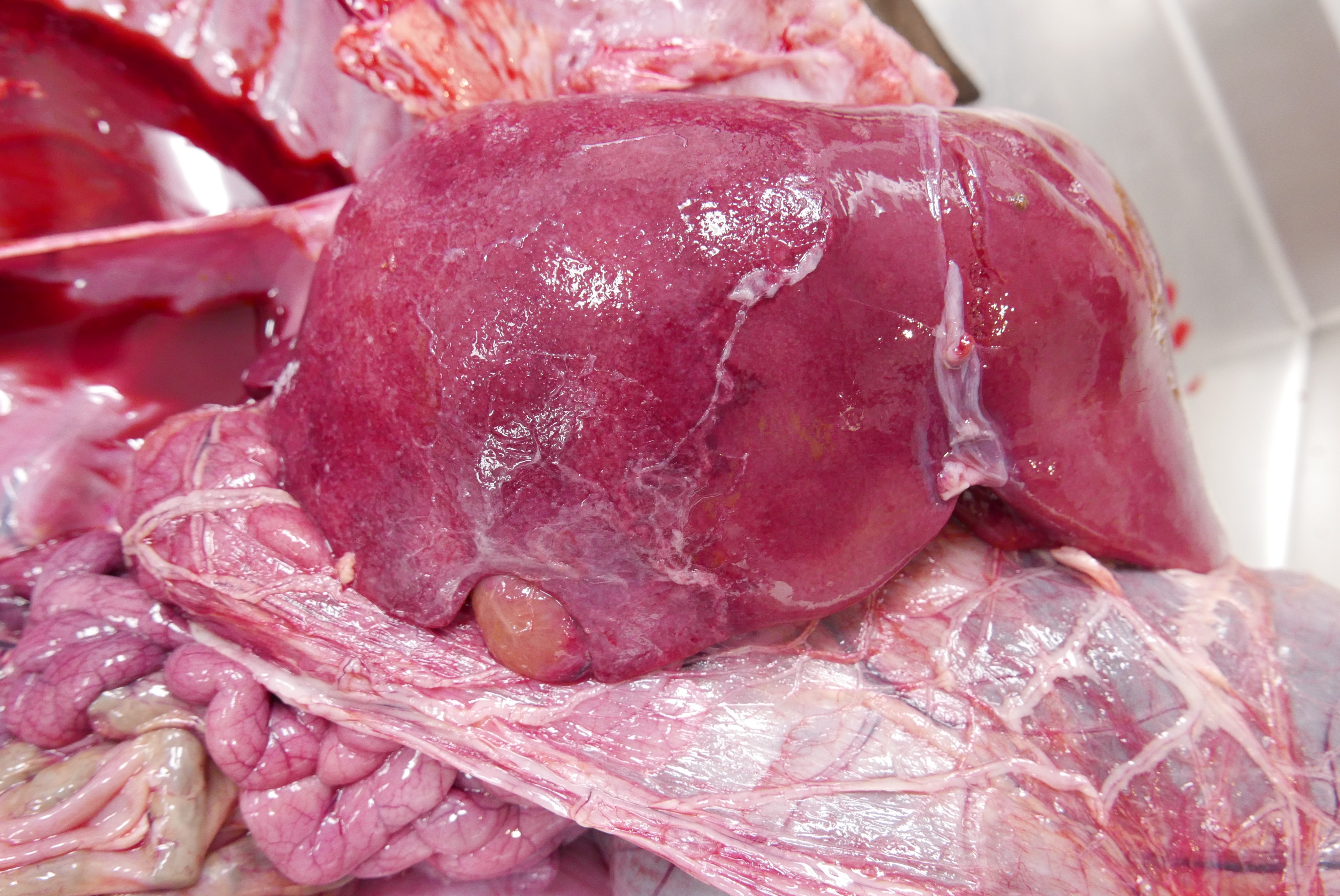
An approximately 10 x 10 cm area of the right lobe of the liver surrounding the gallbladder had a roughened, red to tan granular capsular surface. On cut section, the subtending parenchyma was granular and sharply demarcated from the left lobe. The lungs were diffusely dark red, wet, and heavy.
Laboratory Results:
PCR: A sample of liver was submitted for Chlamydia PCR and was positive. Sequencing identified Chlamydia pecorum.
Microscopic Description:
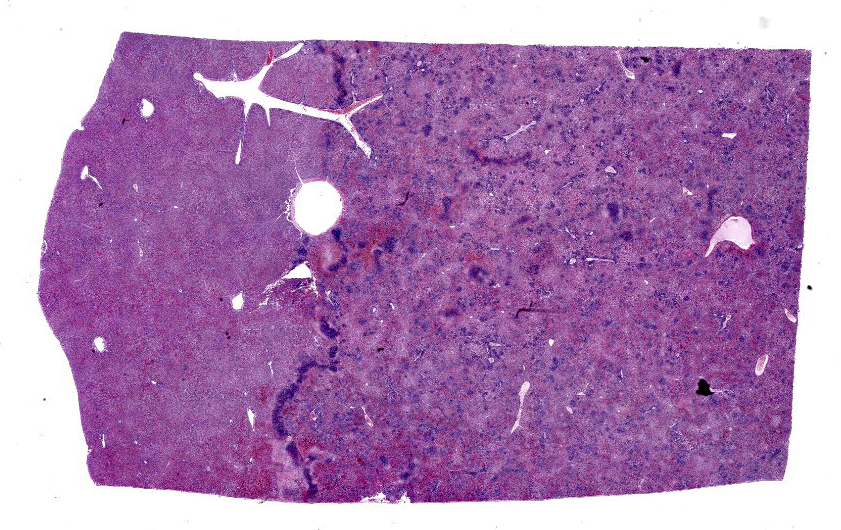
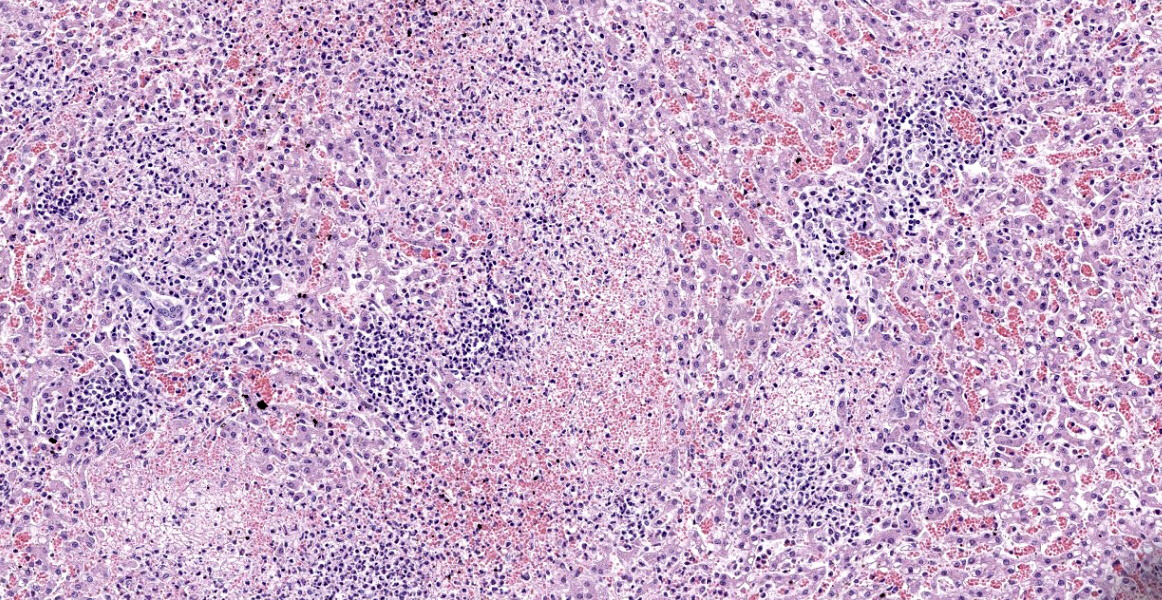
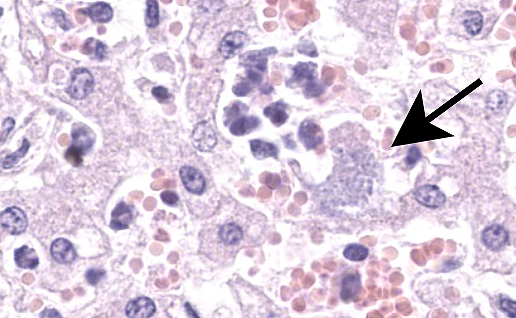
Liver: Within a well-demarcated region of the liver are multifocal distinct and occasionally coalescing foci of neutrophilic inflammation, karyorrhectic debris, and rare multinucleated giant cells that obscure the normal architecture. Neutrophilic aggregates are surrounded by a rim of hepatocytes with a loss of nuclear detail and faint cytoplasmic borders (coagulative necrosis) and acute hemorrhage with fibrin. Kupffer cells adjacent to areas of inflammation and necrosis occasionally have granular amphophilic material in the cytoplasm (coccobacilli). Diffusely, intact hepatocytes have variably sized, well-defined intracytoplasmic vacuoles, and sinsusoids are mildly congested. Multiple portal areas have a mild increase in biliary epithelial cells (ductular reaction).
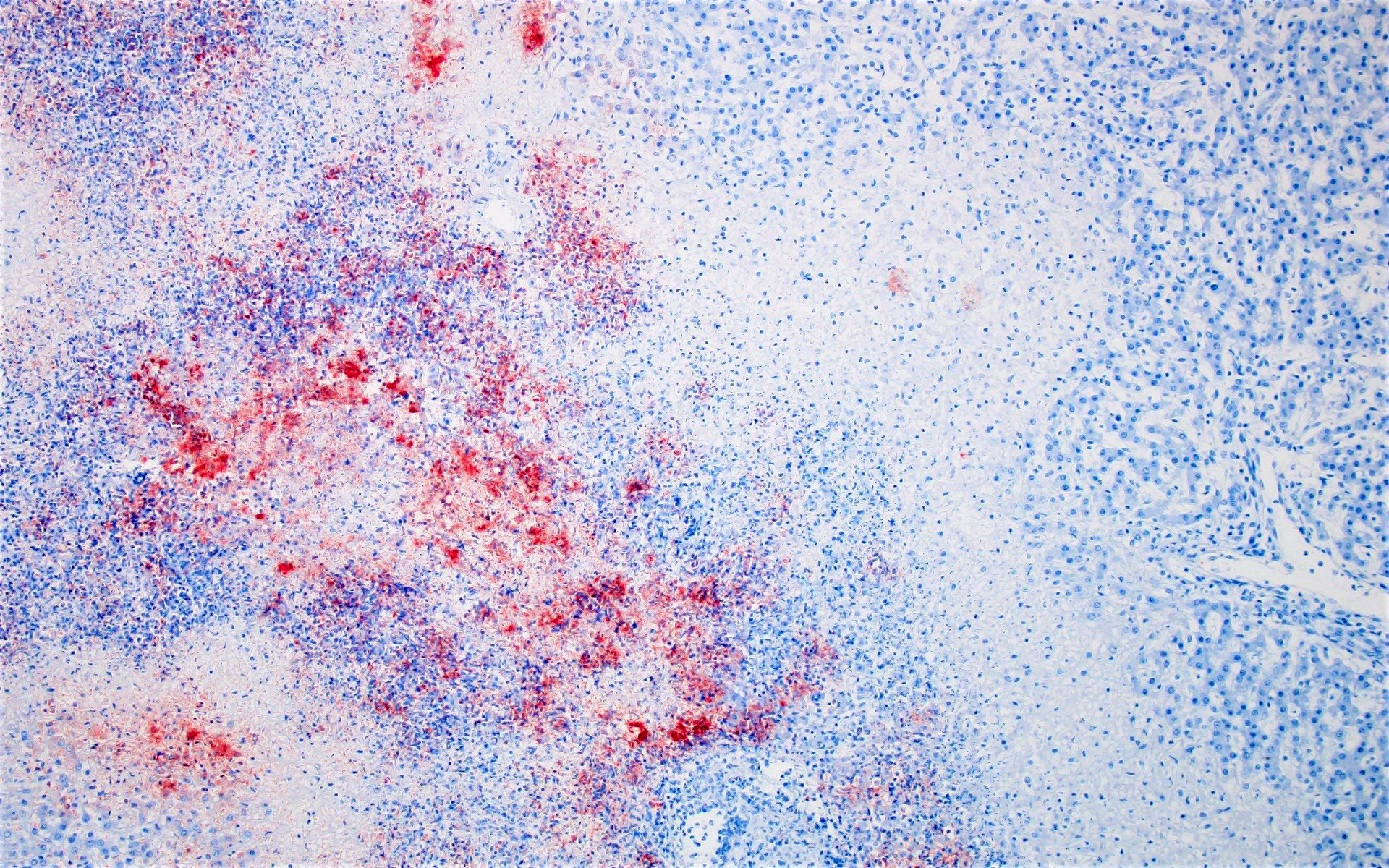
Immunohistochemistry (Chlamydia): Clusters of punctuate, intra-cytoplasmic immunoreactivity is common in areas of necrosis and less common within areas of suppurative inflammation. No immunoreactivity is detected with Coxiella IHC.
Contributor’s Morphologic Diagnoses:
- Liver: Severe, acute, regionally extensive, necrosuppurative hepatitis with intralesional coccobacilli, Chlamydia pecorum
Contributor’s Comment:
Chlamydia pecorum is one of eleven species within the Chlamydia genus, the only genus in the family Chlamydiaceae.12 It is endemic in many populations of cattle and sheep, as detection in the intestines of clinically normal animals is common.1,7 The bacterium has been reported to cause disease in a wide range of species, but koalas, various species of domestic and exotic ruminants, and swine appear to be the most commonly affected. In ruminants, particularly lambs and calves, polyserositis including arthritis, encephalomyelitis, keratoconjunctivitis, mastitis, orchitis, placentitis, endometritis, and pneumonia have been reported.1,6,8,14 Additionally, one study described up to a 48% reduction in gained weight in infected calves suggesting the economic impact extends beyond individuals with clinical disease.10 While less abortogenic than C. abortus, necrotizing placentitis and neutrophilic hepatitis have also been described in goats and sheep that aborted due to C. pecorum infection.5,15 This case is unusual in that the liver was the organ affected in this one-month-old. Most likely, the liver was infected via portal spread from the small intestine. The source of infection in this case is also unknown, as this is the first report of disease caused by C. pecorum at the institution. Spread from nearby domestic animals or direct exposure from subclinically infected conspecifics are both possible.
In koalas, C. pecorum can result in keratoconjunctivitis as well as disease in the urogenital and respiratory tracts resulting in blindness, infertility, or a condition known as ‘wet bottom’ or ‘dirty tail’ due to urine staining of the fur secondary to cystitis and urinary incontinence.1,8 Conjunctivitis due to C. pecorum infection in koalas in Southern Australia has been associated with koala gammaretrovirus infection (KoRV-B).4
Of the Chlamydia spp. that cause disease in animals, C. psittaci is the most well-documented zoonotic species and can be cause mild conjunctivitis (ornithosis) or more severe disease due to myocarditis, encephalitis, or pneumonia.1,12 The zoonotic potential of C. pecorum is less clearly defined.3
A recent review describes the challenges of diagnosing chlamydial diseases in animals.1 While neutrophilic inflammation, necrosis, and basophilic ‘chlamydial’ inclusion bodies are considered histologically characteristic, many infections lack these features or have less specific inflammatory pattern. As a result, immunohistochemistry, histochemical staining, and molecular tests are considered the mainstays of diagnosis of chlamydial diseases in animals. Histochemical stains to highlight the intracellular bacteria via cytology or histology include modified Macchiavello, Giemsa, Castaneda, modified Ziehl-Neelsen, or modified Gimenez.11 Molecular techniques are considered the gold standard for diagnosis due to the inability to isolate Chlamydia spp. on agar plates as they require a host cell for survival. However, care should be taken not to over interpret a positive PCR test as infection with C. pecorum is considered endemic in many ruminants and may be an incidental finding in the absence of associated disease.7 Fluorescent antibody tests and ELISAs also have been developed but are unable to differentiate between various Chlamydia species.11 Immunohistochemistry and PCR were both used in this case to confirm C. pecorum as the causative agent.
Contributing Institution:
Disease Investigations
San Diego Zoo Wildlife Alliance
PO Box 120551
San Diego, CA 92112
http://institute.sandiegozoo.org/disease-investigations
JPC Diagnosis:
Liver: Hepatitis, necrotizing, focally extensive, marked, with intrahepatocytic and intrahistiocytic bacteria.
JPC Comment:
Chlamydia species are obligate intracellular Gram-neagative bacteira with a unique lifecycle which begins as an infectious extracellular elementary body.1,2 Inside the host cell, the bacteria matures within a vacuole into a slightly larger reticulate body, and after several rounds of replication, the bacteria converts back to an elementary body and lyses the cell.1,2 During times of stress, the bacteria may take on an aberrant body phenotype, a non-replicative persistent state.1
While the contributor describes several forms of Chlamydial infection in mammals, the bacteria also causes clinical and subclinical infection in reptiles, amphibians, and fish. In reptiles, clinical disease is characterized by granulomatous inflammation in the heart, lung, liver, and/or spleen.1,2 A recent article in Vet Pathol described an outbreak of Chlamydia in farmed American alligators.2 Over the span of ten days, approximately 100 alligators died acutely. Histologically, these animals had severe necrotizing hepatitis and myocarditis, mild enterocolitis, and splenic lymphoid depletion; most animals also had lymphoplasmacytic interstitial pneumonia and nephritis.2 Animals which died later during the outbreak also had erosive conjunctivitis, keratoconjunctivitis, and mild uveitis; lesions in other systems were less severe than in the animals that died earlier in the outbreak.2 Chlamydial antigen was identified using immunohistochemistry and was particularly high in the liver, heart, spleen, and intestine.2 Based on genetic sequencing and phylogenetic analysis, the authors suspect that the Chlamydia species is closely related to Chlamydia poikilothermis identified in snakes.2
An important differential to consider for this case of well-demarcated, randomly distributed foci of hepatic necrosis in a ruminant is ruminal acidosis with secondary necrobacillosis (rumenitis-liver abscess complex). 13 Ruminal acidosis is caused initially by carbohydrate overload and leads to alterations of microflora, overgrowth of organisms such as Streptococcus bovis, and ruminal atony.13 High levels of acid within the rumen and intestine cause increased osmotic pressure, fluid influx, and systemic hypovolemia. Ultimately, Fusobacterium necrophorum, an opportunistic pathogen which is part of the normal GI flora, invades the ruminal mucosa, causing mucosal necrosis and ulceration, and travels through the portal system causing multifocal random hepatic necrosis.13 While the pattern of necrosis in this case is consistent with necrobacillosis, this case lacks colonies of filamentous bacteria along the margins of necrosis seen with F. necrophorum.
The moderator and conference participants agreed that, while intracellular bacteria were visible on the H&E slide, reaching a definitive diagnosis of Chlamydia pecorum is not possible without further testing (such as with PCR and IHC, as completed in this case.) Other differentials for intracellular bacteria discussed by the participants included Brucella and Clostrium piliforme.
References:
- Borel N, Polkinghorne A, Pospischil A. A review of Chlamydial diseases in animals: still a challenge for pathologists? Vet Pathol. 2018;55:374-390.
- Carossino M, Nevarez JG, Sakaguchi K, et a. An outbreak of systemic chlamydiosis in farmed American alligators (Alligator mississippiensis).Vet Pathol. 2022; 59(5): 860-868.
- Cheong HC, Lee CYQ, Cheok YY, et al. Chlamydiaceae: diseases in primary hosts and zoonosis. 2019;7(5):146.
- Fabijan J, Sarker N, Speight N, et al. Pathological findings in koala retrovirus-positive koalas (Phascolarctos cinereus) from northern and southern Australia. J Comp Path. 2020;176:50-66.
- Giannitti F, Anderson M, Miller M, et al. Chlamydia pecorum: fetal and placental lesions in sporadic caprine abortion. J Vet Diagn Invest. 2016;28:184-189.
- Jelocnik M, Forshaw D, Cotter J, et al. Molecular and pathological insights into Chlamydia pecorum-associated sporadic bovine encephalomyelitis (SBE) in Western Australia. BMC Vet Res. 2014;10:121.
- Li J, Guo W, Kaltenboeck B, et al. Chlamydia pecorum is the endemic intestinal species in cattle while gallinacea, C. psittaci, and C. pneumoniae associate with sporadic systemic infection. Vet Microbiol. 2016;193:93-99.
- Ostfeld N, Islam MM, Jelocnik M, et al. Chlamydia-pecorum-induced arthritis in experimentally and naturally infected sheep. Vet Pathol. 2021;58:346-360.
- Palmieri C, Hulse L, Pagliarani S, et al. Chlamydia pecorum infection in the male reproductive system of koalas (Phascolarctos cinereus). Vet Pathol. 2019;56:300-306.
- Poudel A, Elasser TH, Rahman KhS, et al. Asymptomatic endemic Chlamydia pecorum infections reduce growth rates in calves by up to 48 percent. PLOS ONE. 2012;7:e44961.
- Sachse K, Vretou E, Livingstone M, et al. Recent developments in the laboratory diagnosis of chlamydial infections. Vet Microbiol. 2009;135:2-21.
- Sachse K, Bavoil PM, Kaltenboeck B, et al. Emendation of the family Chlamydiaceae: proposal of a single genus, Chlamydia, to include all currently recognized species. Syst Appl Microbiol. 2015;38:99-103.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th St. Louis, MO: Elsevier. 2016; 40-43.
- Walker E, Lee EJ, Timms P, et al. Chlamydia pecorum infections in sheep and cattle: a common and under-recognised infectious disease with significant impact on animal health. Vet J. 2015;206:252-260.
- Westermann T, Jenkins C, Onizawa E, et al. Chlamydia pecorum-associated sporadic ovine abortion. Vet Pathol. 2021;58:114-122.