Signalment:
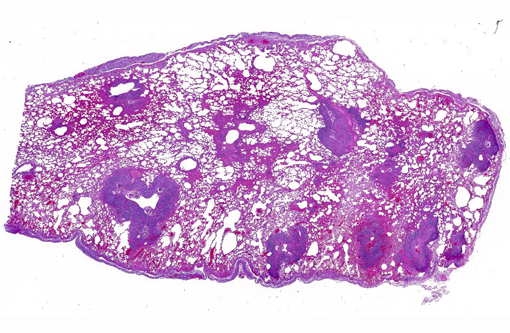
Gross Description:
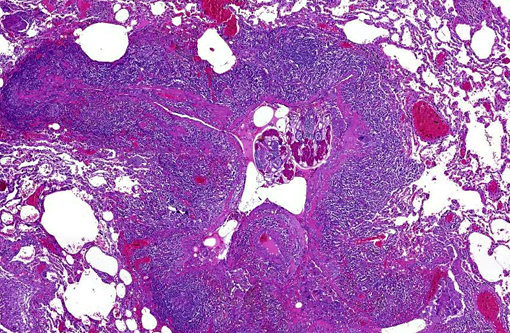
Histopathologic Description:
Morphologic Diagnosis:
1. Bronchiolitis, alveolitis and pleuritis, necrotizing, histiocytic, lymphoplasmacytic, multifocal, severe, chronic with bronchiolectasis, smooth muscle hyperplasia, lymphoid hyperplasia and intraluminal arthropods (Pneumonyssus simicola).Â
2. Vasculitis, multifocal, necrotizing to histiocytic, severe, chronic with multinucleated giant cells; lung.
Lab Results:
Condition:
Contributor Comment:
The exact lifecycle has not been fully elucidated, but adult mites are obligate endoparasites and adults feed on host erythrocytes, lymph and epithelial cells in the lung. Transmission requires close association with infected animals as it is likely through direct contact.Â
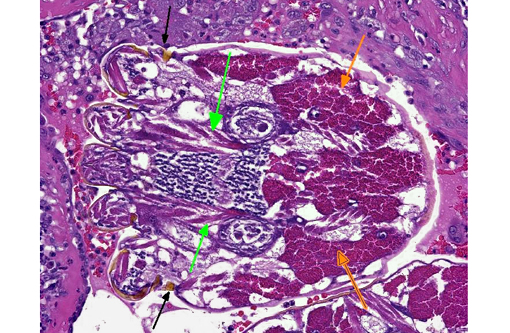
Gross lesions are generally multifocal, round, yellow to tan cystic foci up to several millimeters in diameter within the lung parenchyma. Mites occasionally can be visualized in the center of these lesions with the aid of a dissecting scope. Histopathologic findings typically include granulomatous and eosinophilic inflammation centered on the terminal air passages, pigment-laden macrophages, bronchiectasis, alveolar emphysema, bronchiolar smooth muscle hyperplasia and interstitial fibrosis.(1,4-6) Female mites are most commonly seen in the airways and can be up to approximately 700 μm long with a thin chitinous exoskeleton, jointed appendages and a body cavity with a digestive tract and reproductive tract.(3-5) The characteristic birefringent crystalline pigment, regarded as a metabolite of the female mite, can be present in sections of tissue that lack mites and can be used to make a presumptive diagnosis.
While P. simicola is most frequently described in rhesus macaques, it has also been reported in other macaque species and a baboon.(3) Pulmonary acariasis is not limited to nonhuman primates; there are numerous species of mites that can be found in nasal passages and lungs of other animals including but not limited to Rhinophaga sp. in non-human primates, Pneumonyssoides caninum in dogs, Entonyssus sp. and Entophionyssus sp. in snakes, Cephenemyia sp. in wild cervids, Cytodites nudus in poultry, and Sternostoma tracheacolum in Gouldian finches.
JPC Diagnosis:
1. Lung: Bronchiolitis, granulomatous and necrotizing, chronic, multifocal, severe, with bronchiolar smooth muscle hyperplasia, bronchiolectasis and intrabronchiolar arthropods and mite pigment.Â
2. Pleura: Serositis, granulomatous, multifocal, moderate, with epithelial hyperplasia.Â
3. Subpleural vasculature, smooth muscle: Hyperplasia, diffuse, mild to moderate.Â
Conference Comment:
The term acariasis equates with a mite infection and is derived from the Order Acari in which all mites are classified. While most mite infections are localized to the skin, there are at least ten species of lung mites which infect the lungs of Old World monkeys, all of the genus Pneumonyssus.(7)
Conference participants debated on possible causes of the evident granulomatous inflammation with multinucleated cells lining the outside of the pleura in this case. Most agreed with the contributors suspicions of a secondary pneumothorax, possibly a sequela of ruptured mite houses. Additional Gram, fungal and acid-fast stains did not elucidate any additional infectious organisms. Without definitive causal evidence, we elected to separate out the diagnoses of serositis and the prominent smooth muscle hyperplasia of subpleural vessels.Â
As nicely described by the contributor, mite pigment is present in abundance in many sections. This is a golden brown to black pigment usually found within macrophages. This pigment does not contain carbon or melanin, but rather is iron-positive and likely the result of breakdown and excretion of the hosts blood proteins.(7)
References:
1. Abbott DP, Majeed SK. A survey of parasitic lesions in wild-caught, laboratory-maintained primates: (rhesus, cynomolgus, and baboon). Veterinary Pathology. 1984;21(2):198-207.
2. Andrade, MCR, Marchevsky, RS. Histopathologic findings of pulmonary acariasis in a rhesus monkeys breeding unit. [Aspectos histopatol³gicos da acar+�-�ase pulmonar em uma cria+�-�+�-�o de maca-cos rhesus.] Revista Brasileira de Parasitologia Veterin+�-�ria. 2007;16(4):229-234.
3. Cogswell F. Parasites of non-human primates. In: Baker DG, ed. Flynns Parasites of Laboratory Animals. 2nd ed. Ames, Iowa: Blackwell Publishing; 2007:716-717.
4. Innes JR, Colton MW, Yevich PP, Smith CL. Lung mites; pulmonary acariasis as an enzootic disease caused by Pneumonyssus simicola in imported monkeys. The American Journal of Pathology. 1954;30(4):813-35.
5. Ogata T, Imai H, Coulston F. Pulmonary acariasis in rhesus monkeys: electron microscopy study. Experimental and Molecular Pathology. 1971;15(2):137-47.
6. Osborn K, Lowenstein L. Respiratory diseases. In: Bennett B, Abee C, Henrickson R, eds. Nonhuman Primates in Biomedical Research: Diseases. 1st ed. Vol. 2. San Diego, CA: Academic Press; 1998:302-303.Â
7. Strait K, Else JG, Eberhard ML. Parasitic diseases of nonhuman primates. In: Abee CR, Mansfield K, Tardiff S, Morris T, eds. Nonhuman Primates in Biomedical Research: Diseases. 2nd ed. Vol. 2. San Diego, CA: Academic Press; 2012:268-270.