Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 22
April 18th, 2018
CASE II: P636-14 (JPC 4066540)
Signalment: 8-week-old, intact, female, Braque francais (French pointer) (Canis familiaris), canine.
History: This puppy was presented to a veterinary clinic for a sudden onset of drooling (sialorrhea), vomiting, tremors and seizures. Due to the severity and rapid evolution of the clinical signs, the owner elected for euthanasia without any further investigation. The dog was submitted to our diagnostic laboratory and a complete necropsy was performed.
Gross Pathology: The dog was in good body condition and there were no remarkable findings on gross examination.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): The dog’s brain tested negative for rabies (immunoperoxidase) and herpesvirus (PCR à panherpesvirus/DNA polymerase on FFPE tissue).
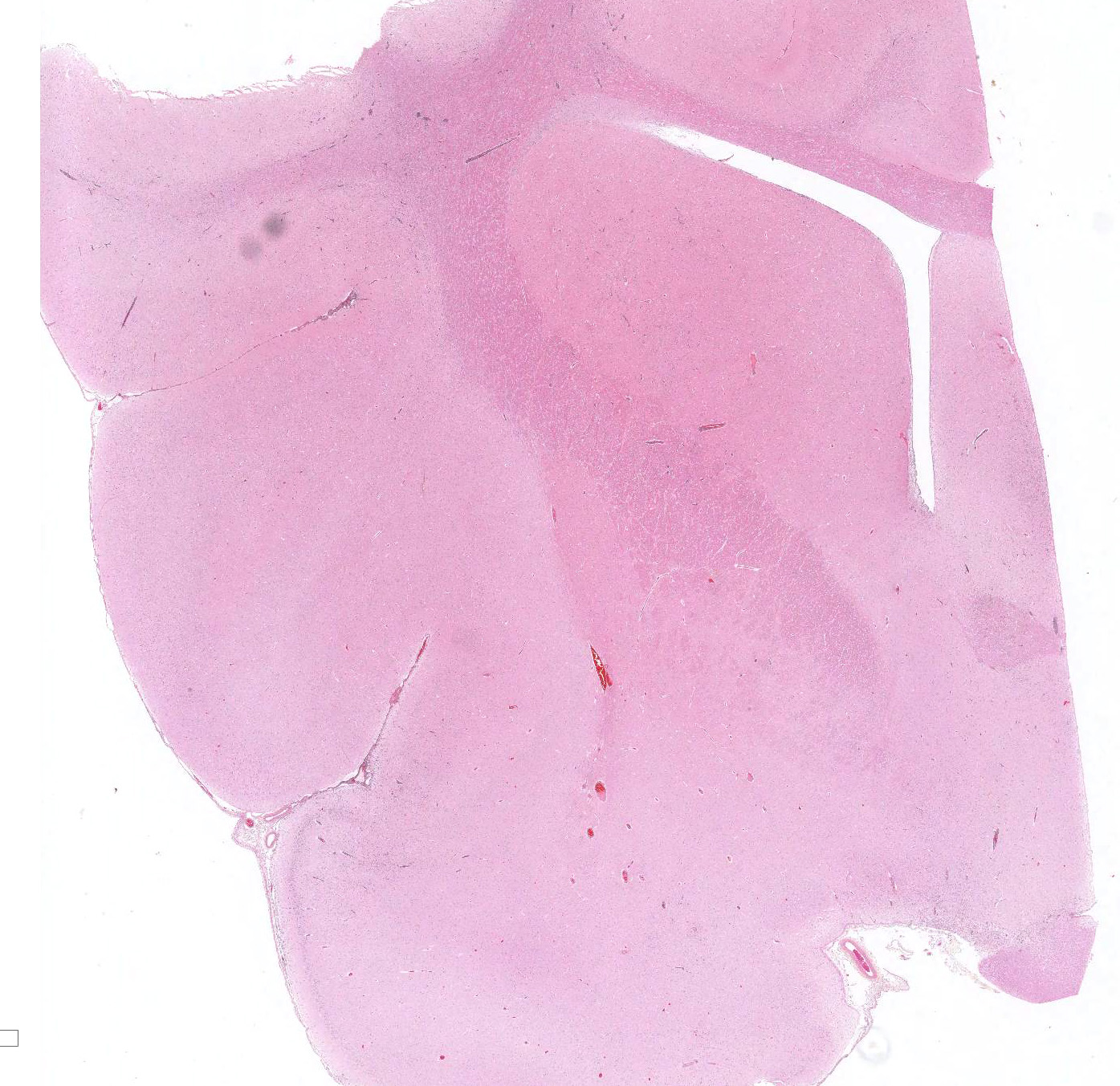
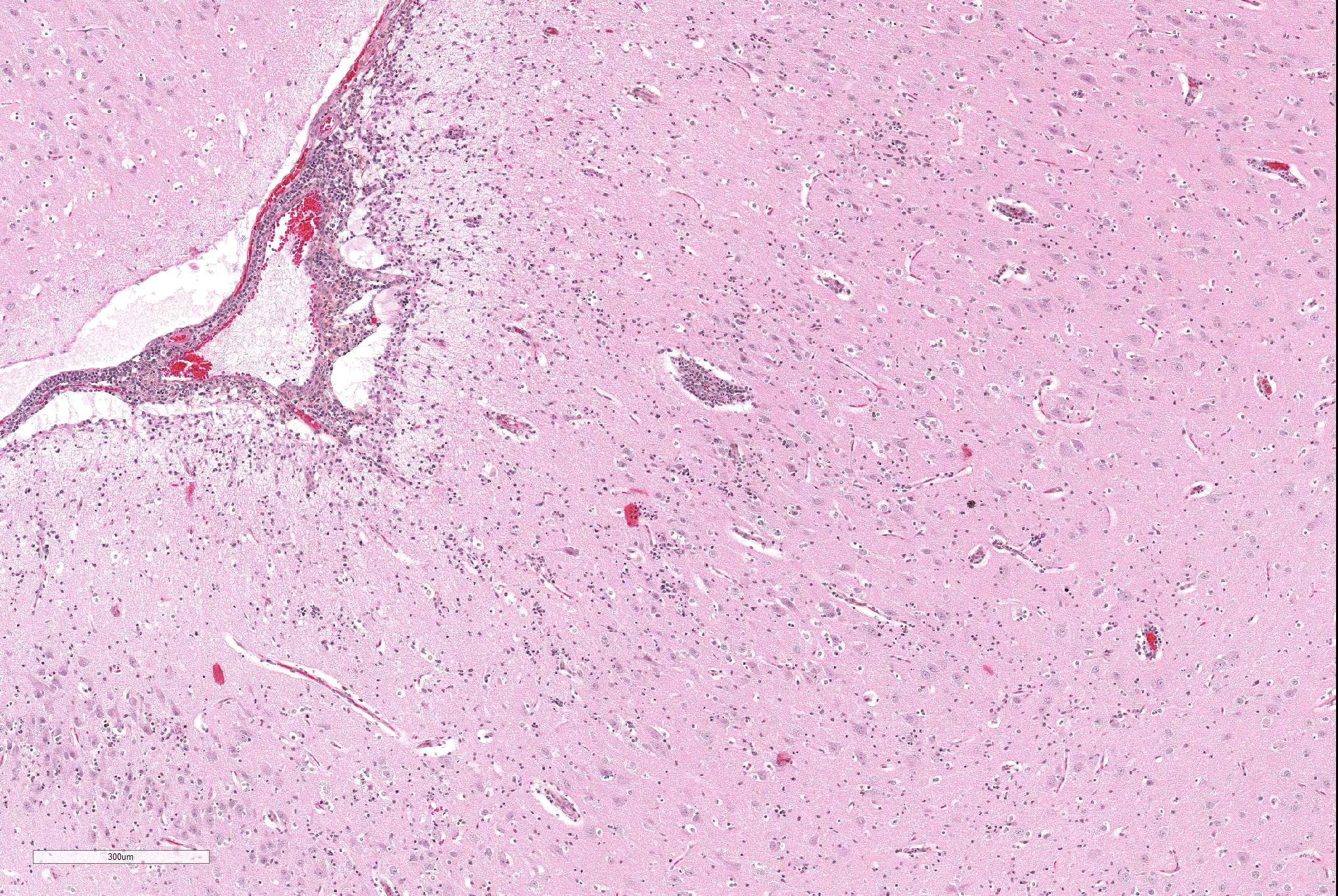
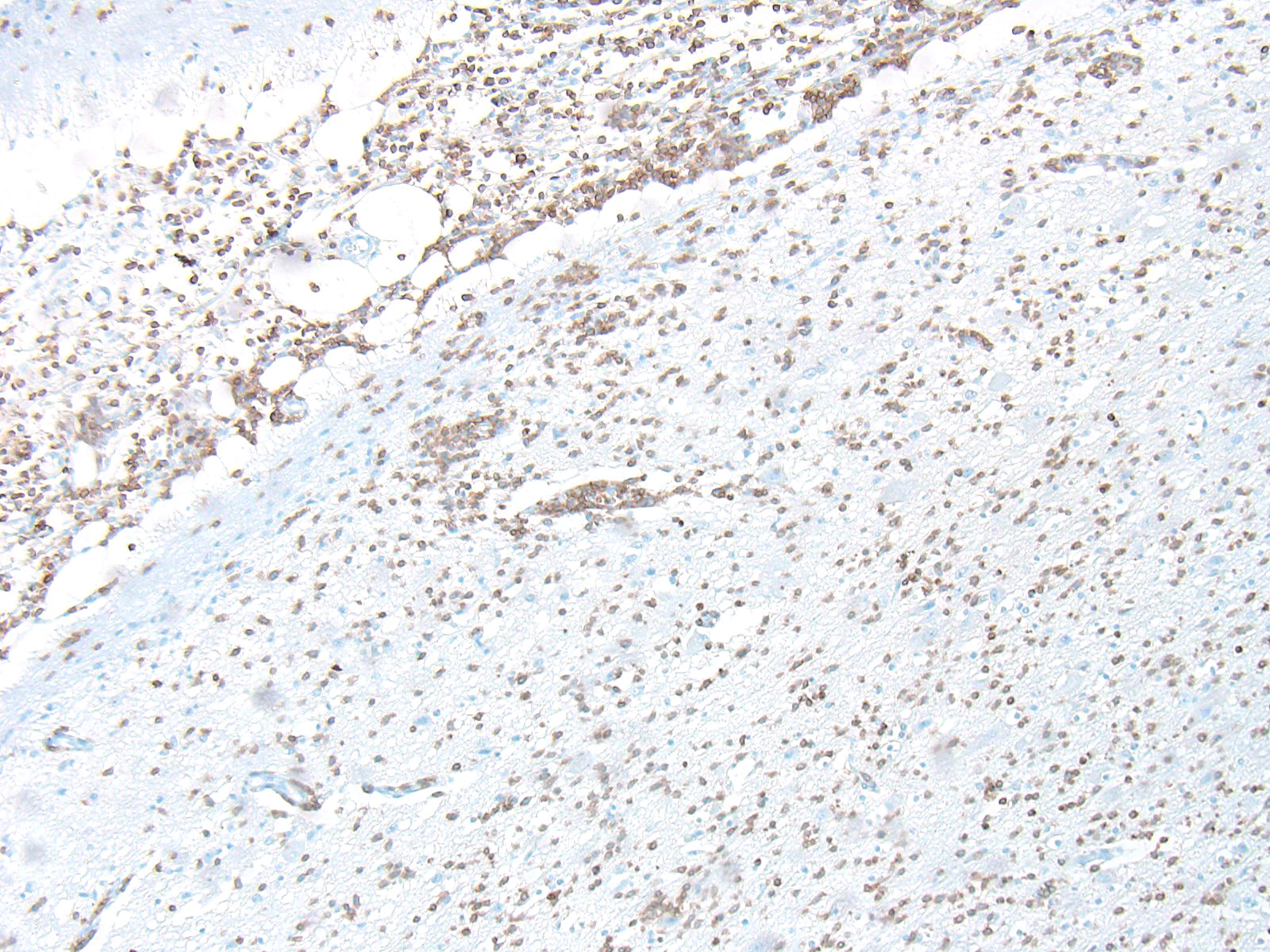
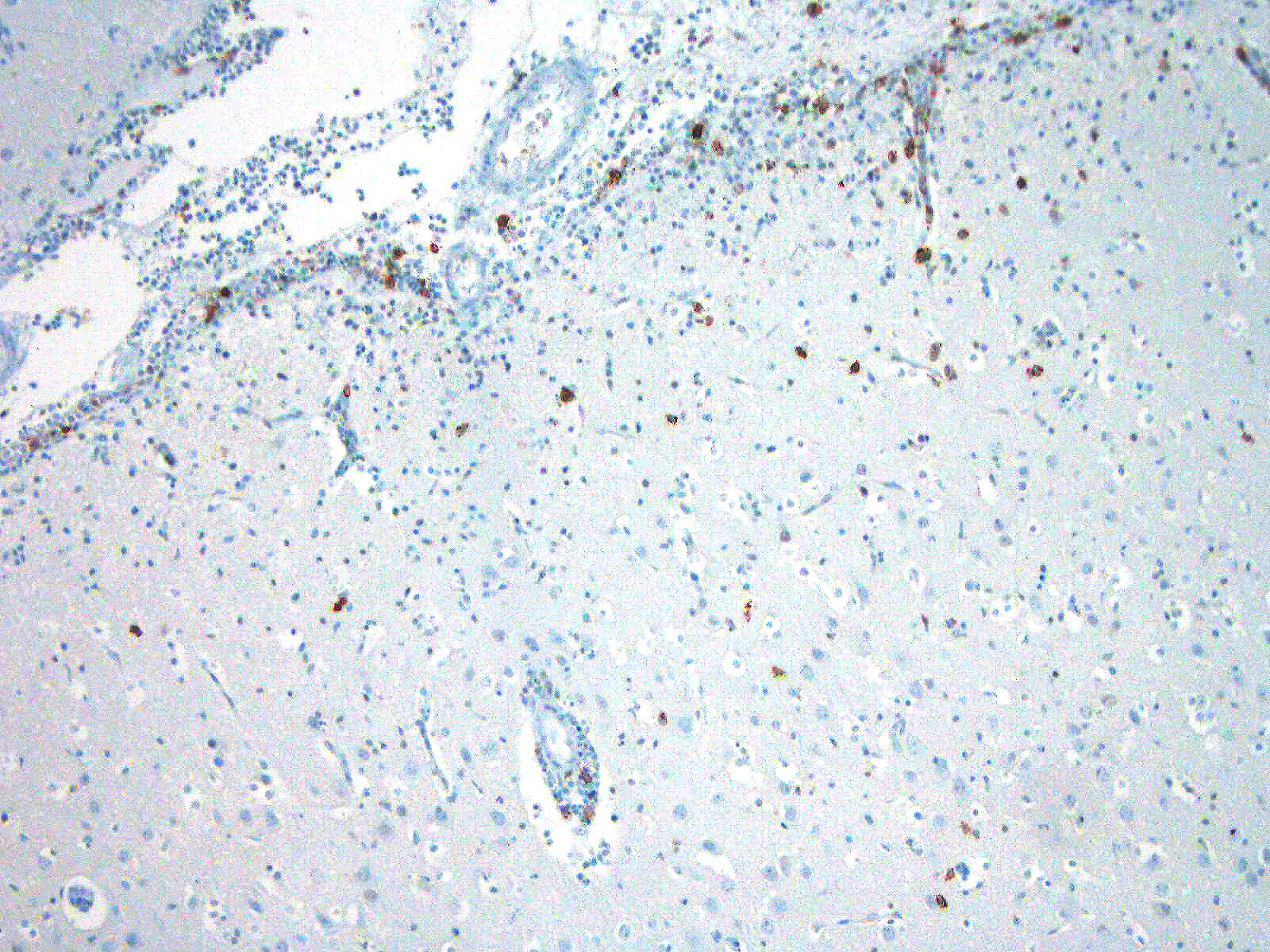
Microscopic Description: Similar lesions were observed throughout the brain and the cranial portion of the cervical spinal cord, with relatively minor variations in intensity. The submitted sections are from the cerebrum. Multifocally in the neuroparenchyma and, to a lesser degree, the leptomeninges (subarachnoid space), there is a population of relatively monomorphic lymphoid cells that have a mainly perivascular and, in the neuroparenchyma, vascular distribution (Fig.1). In the neuroparenchyma, these cells are located in the Virchow-Robin spaces (up to 8 cells thick) and/or the vascular walls in both white and grey matter; in the latter case, there is sometimes associated edema, fibrin and/or erythrocytes (Figs 1 and 2). Multifocally, especially around affected blood vessels, the neuropil has an increased cellularity due to the presence of apparently similar lymphoid cells and possibly glial cells (some of which are reactive) (Fig.1). The lymphoid cells are small (nuclei ≤ 1.5 RBC), and are characterized by scant eosinophilic cytoplasm, a round to oval nucleus with finely stippled chromatin. There are several to numerous cells, interpreted as lymphoid cells, that are karyorrhectic or sometimes pyknotic (apoptosis). Anisocytosis and anisokaryosis are minimal, and no mitoses were detected. Immunohistochemistry (IHC) for CD3 and CD79a was performed on sections of the brain. The overwhelming majority (> 99%) of lymphoid cells, including in the hypercellular neuropil, were strongly positive for CD3 (Figs.3 and 4), with very rare cells (< 1%) positive for CD79a. There were no significant changes in other organs examined, including the eyes.
Contributor’s Morphologic Diagnosis:
Primary CNS T-cell lymphoma (PCNSL)
Contributor’s Comment: Although a diagnosis of viral mononuclear/lymphocytic meningoencephalomyelitis was initially considered and investigated (CDV and herpesvirus), primary CNS T-cell lymphoma was the final diagnosis based on the morphologically monomorphic nature of the lymphocytic infiltrate (including the absence of plasma cells) that was confirmed to be almost purely of T cell nature, and its angiocentric nature; although not assessed by IHC, macrophages did not seem to be present, at least in significant numbers, and glial nodules were not observed. Assessment of T-cell clonality by either T-cell antigen receptor gene rearrangement analysis (PCR) or X-chromosome inactivation pattern (XCIP) analysis was not performed. Demonstration of clonality would have further supported our diagnosis, although it is not totally confirmative; the XCIP technique has more limitations, especially with regards to gender (females) and hematopoietic neoplasms.9
Primary CNS lymphomas (PCNSL) are uncommon to rare neoplasms defined as non-Hodgkin lymphomas that are confined, at least initially, to the CNS and/or the eye.1,3,12,13 In the CNS, they can involve the neuroparenchyma and/or the meninges. Primary ocular lymphomas included in the PCNSLs in humans are vitreoretinal lymphomas (PVRL); 65-90% of patients with PVRLs develop lymphoma in the CNS.2 In contrast to PCNSLs, secondary CNS lymphomas represent a metastatic process from a lymphoma outside the CNS, and are more common.1,3,4,13 In humans and cats, 5% of patients with systemic lymphomas have CNS involvement, predominantly in the leptomeninges.8,13 PCNSLs are neoplasms mainly seen in humans, dogs and, to a lesser degree cats, but they have also been described in ruminants, a dolphin and a harbor seal;1,3-5,7,8,12,13 there is a probable case reported in a horse.11 In humans and dogs, the reported incidence is about 3% of intracranial neoplasms; in humans they account for 1-2% of all non-Hodgkin lymphomas. In cats, most PCNSLs involve the spinal cord.4 In animals, the majority of PCNSLs are of T-cell lineage, in contrast with humans in which 80-95% are large B-cell lymphomas which differ from their systemic counterparts with regards to behavior and treatment.1,3,7,12 The etiology is unknown in both animals and humans, except in cats in which FeLV is often involved and in immunocompromised humans in which the Epstein-Barr virus (EBV) plays a major role (EBV has not been associated with PCNSL in immunocompetent human patients).1,12 Most PCNSL cases have gross lesions and the diagnosis of lymphoma is straightforward on microscopic examination, but in a few reported cases in cats and dogs,4,5,7,8 there were no conspicuous masses and histopathology could not readily differentiate between lymphoma and mononuclear/ lymphocytic inflammation. In human PCNSLs, neoplastic lymphocytes in the neuroparenchyma characteristically invade walls of small blood vessels, accumulate in perivascular spaces and spread into the neuropil.1,3
In the present case, the age of the animal, absence of gross lesions and, histologically, absence of “masses” of lymphoid cells, atypia and mitosis initially led to a diagnosis of mononuclear (non-suppurative) meningoencephalomyelitis of probable viral origin. Other infectious diseases (e.g. neosporosis), granulomatous meningoencephalitis (GME) and necrotizing meningoencephalitis (NME) were included in the initial differential diagnosis, but the histopathologic findings were not consistent with these conditions. Based on aforementioned findings (homogenous infiltrate, angiocentrism and IHC), some published veterinary cases and a consultation with a veterinary neuropathologist, we finally concluded to a PCNSL. An equine case with similarities to our case was termed “lymphoproliferative disease with features of lymphoma in the CNS”.11 In human pathology, small-cell lymphomas have been distinguished from mononuclear encephalitides by two morphologic criteria: 1) encephalitis generally has a more polymorphous infiltrate with at least some plasma cells, and 2) vascular invasion by lymphocytes, characteristic in lymphomas, is not a feature of encephalitis.3 The age of the animal is also not typical of canine lymphomas which are mostly seen in middle-aged dogs, even though they have been seen in puppies as young as 4 months. In the bovine species, lymphomas have been reported in aborted fetuses and neonatal calves.6
JPC Diagnosis: Cerebrum: Meningoencephalitis, lymphocytic, multifocal to coalescing, moderate, Braque francais (French pointer) (Canis familiaris), canine.
Conference Comment: As described by the contributor above, primary central nervous system (CNS) lymphoma is rare in humans and animals. In humans the majority are large B-cell variants, whereas, in animals most of the reported cases have been T-cell variants. Microscopically, the diagnosis of lymphoma is challenging because the perivascular and periventricular distribution of neoplastic cells may be confused with an inflammatory response (e.g. viral encephalitis). However, inflammatory responses often have several other cell types depending on chronicity, exhibiting neutrophilic infiltrates initially, followed by plasma cells, lymphocytes, and potentially histiocytes as the infection progresses. In humans, an atypical variant of CNS lymphoma has been described termed “lymphomatosis cerebri” which is characterized by diffuse infiltration of deep cerebral white matter by individualized lymphoma cells with no mass formation. Another differential which has been reported in humans and domestic animals is lymphomatoid granulomatosis, a rare primary pulmonary lymphoproliferative disease which has had CNS involvement described in late stages. This is characterized microscopically by angiocentric and angiodestructive atypical lymphoid cells with rare binucleate and multinucleate cells.10
The conference attendees discussed the alternative diagnoses, given the clinical presentation (young puppy). Conference attendees reviewed common viral encephalitides (distemper, alphaviruses, West Nile virus, rabies, canine herpesvirus-1 ) with their microscopic characteristics. It was noted that immunohistochemistry for B cells or tests for T-cell clonality were not performed as part of the workup. Several immunohistochemical stains were run by the JPC in this case to further classify the cell type in these specimens. While the majority of cells exhibited strong cytoplasmic positivity for T-cells, a number of lymphocytes both in perivascular and parenchymal locations stained positively for CD20, a B-lymphocyte marker. PARR testing was discussed as an important next step to determine if this represents a clonal expansion of T cells. Based on the morphologic appearance of the lesion, the relatively frequency of both subclinical viral infections in puppies versus the frequency of primary T-cell lymphomas (the age of this puppy notwithstanding), the presence of B-cells within the lesion, and the absence of evidence of clonality, the moderator and attendees favored a diagnosis of inflammatory disease in this case rather than T-cell lymphoma.
Contributing Institution:
Faculty of veterinary medicine,
Université de Montréal,
St-Hyacinthe, Quebec, Canada
http://www.medvet.umontreal.ca
References:
- Arbelo M, Espinosa de los Monteros A, Herraez P, et al. Primary central nervous system T-cell lymphoma in a common dolphin (Delphinus delphis). J Comp Path. 2014; 150:336-340.
- Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16:1589-99
- Ellison D, Love S, Chimelli L, et al. Primary CNS lymphomas. In: Neuropathology: a reference text of CNS pathology. Second edition. Mosby, Philadelphia, PA, 2004: 689-694.
- Fondevila D, Vilafranca M, Pumarola M. Primary central nervous system T-cell lymphoma in a cat. Vet Pathol. 1998;35:550-3.
- Guil-Luna S, Carrasco L, Gómez-Laguna J, et al. Primary central nervous system T-cell lymphoma mimicking meningoencephalomyelitis in a cat. Can Vet J. 2013;54:602-5.
- Jacobs RM, Messick JB, Valli VE. Tumors of the hemolymphatic system. In: Tumors in domestic animals. Fourth Edition. Iowa State Press, Ames, IA, 2002:119-198.
- Kim NH, Ciesielski T, Kim JH, et al. Primary central nervous system B-cell lymphoma in a young dog. Can Vet J. 2012; 53:559-564.
- Long SN, Johnston PE, Anderson TJ. Primary T-cell lymphoma of the central nervous system in a dog. J Am Vet Med Assoc. 2001;218:719-22.
- Mochizuki H, Goto-Koshino Y, Takahashi M, Fujino Y, Ohno K, Tsujimoto H. Demonstration of the cell clonality in canine hematopoietic tumors by X-chromosome inactivation pattern analysis. Vet Pathol. 2015 Jan;52(1):61-9
- Morita T, Kondo H, Okamoto M, Park CH, Sawashima Y, Shimada A. Periventricular spread of primary central nervous system T-cell lymphoma in a cat. J Comp Pathol. 2009;140(1):54-58.
- Morrison LR, Freel K, Henderson I, et al. Lymphoproliferative disease with features of lymphoma in the central nervous system of a horse. J Comp Pathol. 2008;139:256-61.
- Nigo M, Richardson S, Azizi E, et al. Ventriculitis Caused by Primary T-Cell CNS Lymphoma in an Immunocompetent Patient. J Clin Oncol. 2014 Nov 17 (Epub ahead of print).
- Vandevelde M, Higgins RJ, Oevermann A. Neoplasia. In: Veterinary Neuropathology: essentials of theory and practice. First edition. Ames, Iowa: Wiley-Blackwell, 2012: 129-156.