CASE 3: 17-0646 (4120045-00)
Signalment:
Female Yucatan mini pigs (Sus scrofa), 7-8 months of age, 20kg.
History:
Ten female, 7-8 month old, ~20kg, Yucatan mini-pigs were purchased from a single vendor. Left ulnar nerve transection was performed per protocol 26 days after arrival. All pigs recovered uneventfully from the experimental procedure. All pigs were scheduled to be housed 6 months post-surgery with no other procedures performed. Approximately two months post-procedure, the first lesions appeared in the first pig. Two other pigs also developed similar lesions, one of which recovered from the first instance and developed them a second time. The animal in this report was the third to develop the lesions and appeared to be the worst affected. All three pigs were treated with nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide ointment. In addition to the ointment, the pig in this case was initially treated with silver sulfadiazine ointment.
Gross Pathology:
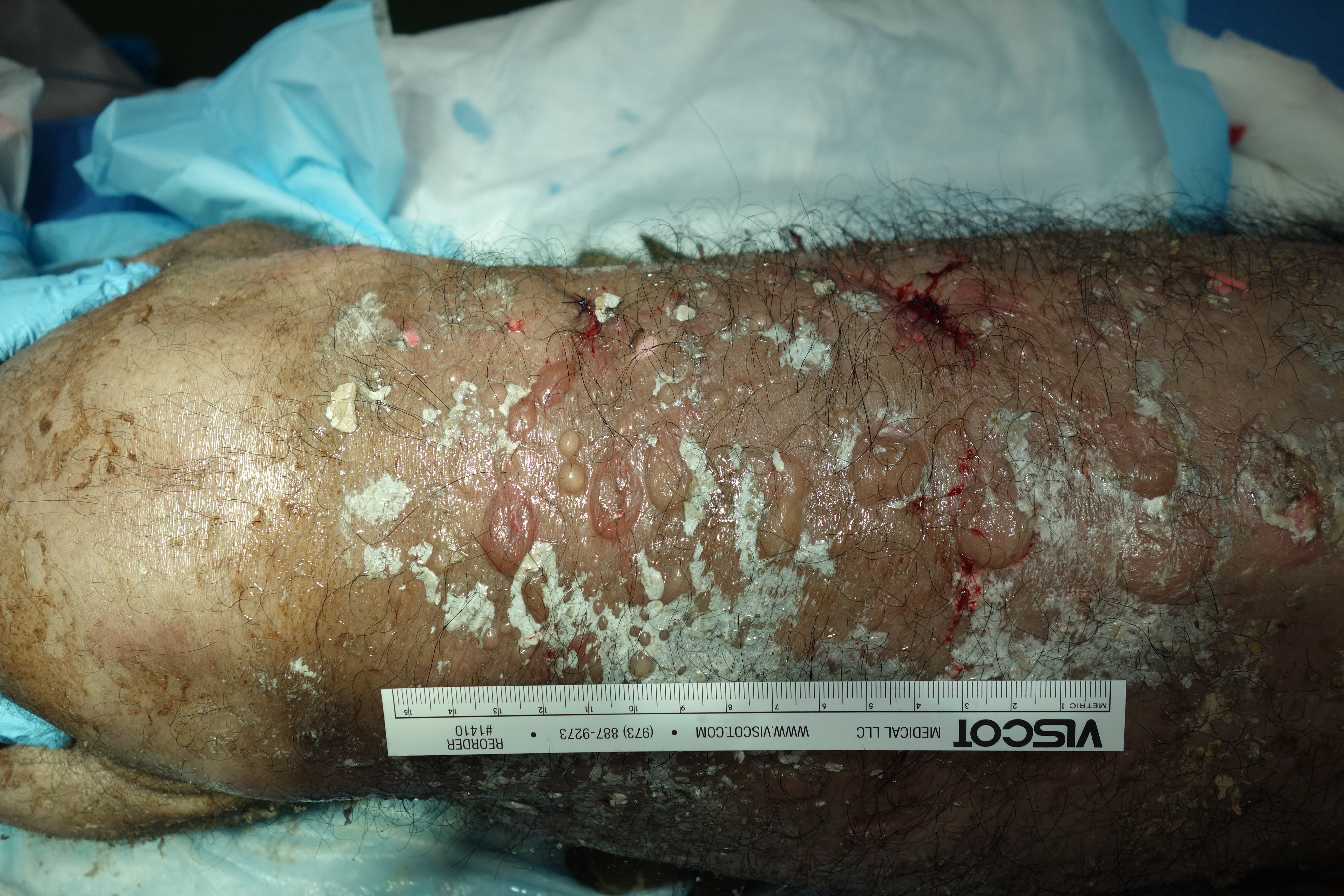
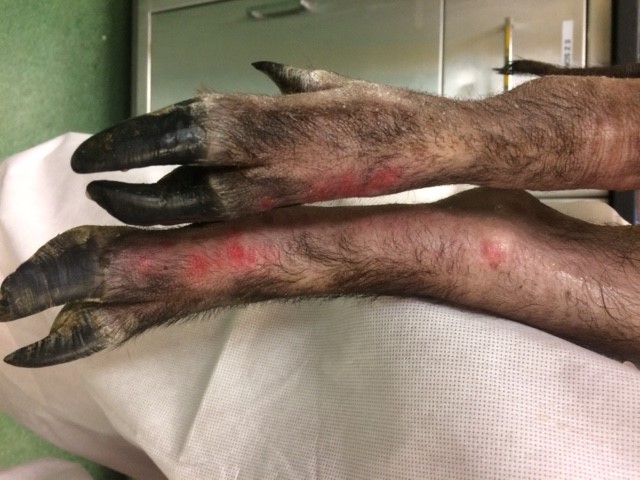
Multifocal, 5 mm to 2 cm diameter, vesicles filled with thick clear viscous fluid were concentrated over the dorsal back with extension down the dorso-cranial aspect of both hind legs to within 1 cm of the coronary band. The vesicles ruptured easily resulting in round ulcerated lesions. Two 10 mm punch biopsies were taken, one from the wall of a large vesicle, the second of an entire intact small vesicle.
Laboratory results:
N/A
Microscopic description:
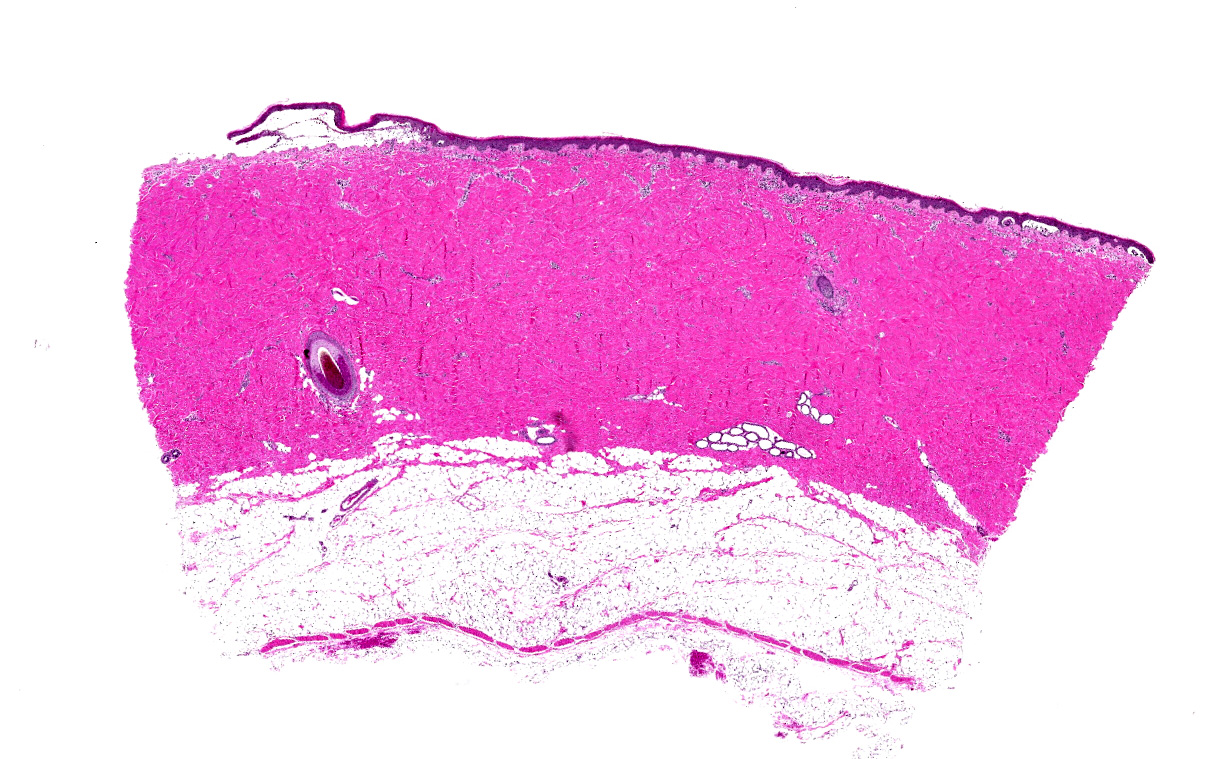
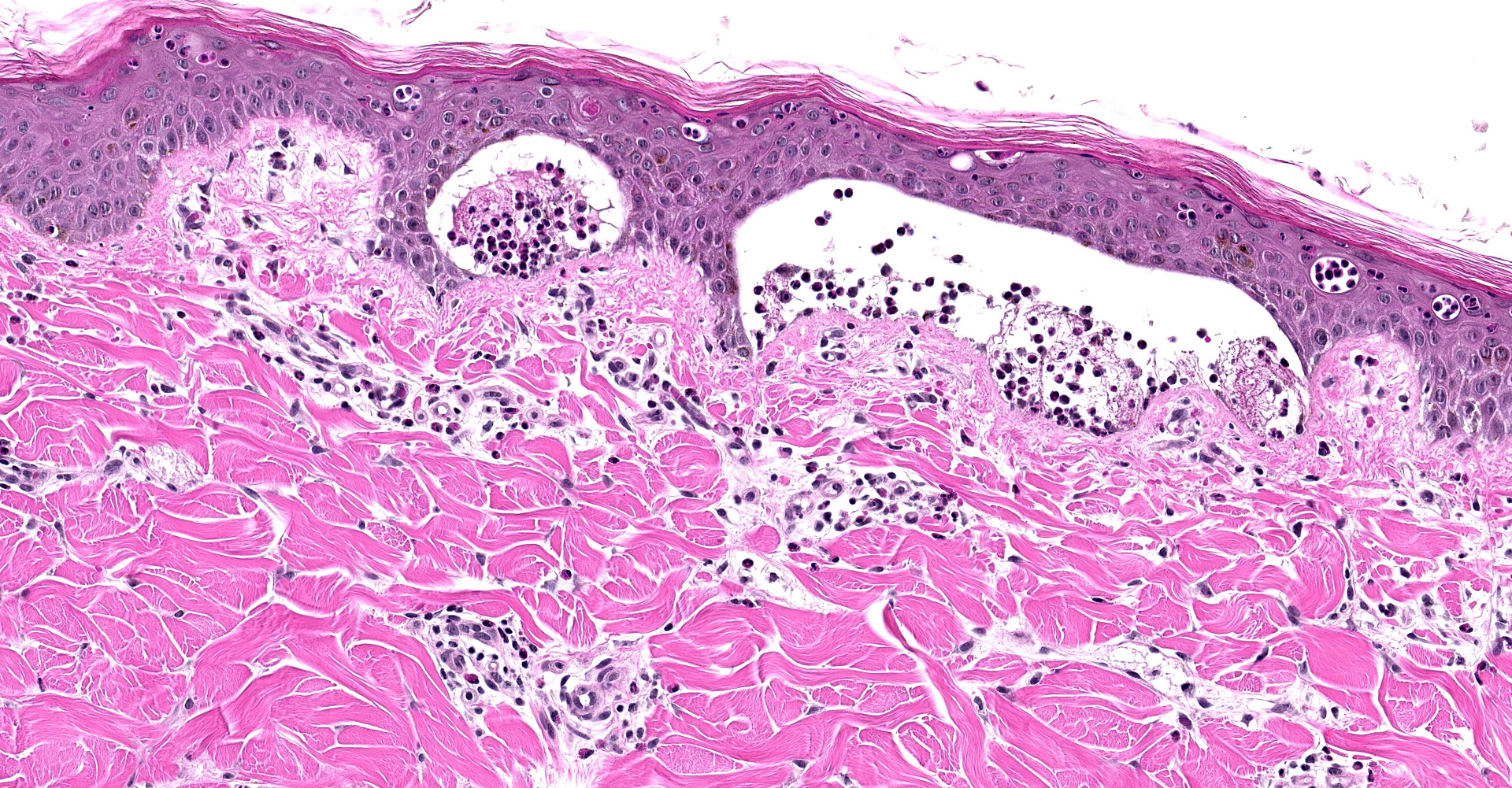
Haired skin, dorsum: Multifocally there are vesicles measuring up to 2 cm in length that elevate the sub basilar space below the epidermis up to 4 mm and contain numerous viable and degenerate eosinophils, lesser lymphocytes, few Mott cells, intact erythrocytes, fibrin, and wispy degenerated collagen, along with eosinophilic proteinaceous fluid, and increased clear space (edema). Frequently vesicular contents indent or extend into the overlying epidermis or form small 1 mm pustules within the stratum spinosum. Affected keratinocytes are shrunken, hypereosinophilic with pyknotic nuclei (necrotic) or vacuolated (degenerate). The overlying stratum corneum is intact and there is mild orthokeratotic hyperkeratosis. The underlying dermis is superficially smudged and contains few perivascular, previously described inflammatory cells.
Contributor's morphologic diagnosis:
Haired skin (dorsal back): Dermatitis, vesicular and sub basal, multifocal, with mild perivascular inflammation.
Contributor's comment:
Autoimmune dermatoses occur when aberrant T or B cells attack self-antigens resulting in a variety of clinical presentation,3 or when autoantibodies attack antigens.4
A recent proposal by Olivry4 has been made to separate diseases based on one of these two mechanisms of action in order to facilitate treatment methodologies that target specific branches of the immune system. Previously, animal autoimmune disorders were separated into "vesiculous" or "bullous" (i.e., the blister-forming pemphigus and pemphigoid variants) and "non-bullous" diseases (i.e., discoid and systemic lupus erythematosus).
Inciting factors include but are not limited to drug therapy, paraneoplastic syndrome, tissue injury, infectious diseases, genetic makeup, and other autoimmune diseases.3
Numerous pathological processes are suspected, which include3:
- Epitope spreading: targets of autoimmune response drift to include other epitopes on the same protein or nearby proteins of the same tissue, resulting in regional variation of the same disease
- Drug induced: Haptenization of keratinocyte antigens makes them immunogenic
- Environmental influences: alters DNA methylation, which in turn, alters gene expression.
- UV-light: induces keratinocyte ICAM-1 and induction of pro-inflammatory cytokines.
- Infectious: structural proteins of agents mimic host tissue proteins
- Gonadectomy: Despite the advantages, neutering is associated with increased risk for certain autoimmune disorders including pemphigus complex6
In people, and potentially animals, blistering dermatoses share the same pathogenic mechanisms as multiorgan diseases. Clinicians should consider potential systemic involvement, as well as the autoimmune and inflammatory conditions that are frequently associated with it.8
- In paraneoplastic pemphigus/ paraneoplastic autoimmune multiorgan syndrome, the internal organs (particularly the lungs) are affected by the autoimmune injury
- Pemphigus erythematosus is associated with lupus erythematosus
- Bullous pemphigoid is associated with neurologic disease, possibly caused by cross-reactivity of the autoantibodies with isoforms of bullous pemphigoid antigens expressed in the skin and brain
- Anti-laminin 332 pemphigoid shows an increased risk for adenocarcinomas
- Patients with anti-p200 pemphigoid often suffer from psoriasis
- Bullous systemic lupus erythematosus is part of the clinical spectrum of systemic lupus erythematosus, a prototypic autoimmune disease with multisystem involvement
- ?5 chain of type IV collagen blistering disease is associated with nephropathy
- Pemphigoid gestationis is associated with pregnancy or trophoblastic tumors
- Linear IgA dermatosis is associated with inflammatory bowel disease
- Dermatitis herpetiformis is associated with celiac disease
- Epidermolysis bullosa acquisita is associated with inflammatory bowel disease
Contributing Institution:
Walter Reed Army Institute of Research/Naval Medical Research Center or WRAIR/NMRC
JPC diagnosis:
Haired skin: Dermatitis, vesicular and eosinophilic, sub-basal, multifocal moderate to severe, with eosinophilic pustules.
JPC comment:
The contributor provides a succinct summary of bullous pemphigoid. While this disease has common features across species, the specific antigens attacked differ. In humans, antibodies attack BP antigen 1 (also called BP230) and BP antigen 2 (BP180), most often targeting the components of hemidesmosomes of the skin. In animals, only BPAG2 has been identified as a target of autoimmunity.3
In human epidemiological studies, a variety of factors may be risk factors for developing bullous pemphigoid. One of the most interesting risk factors with relevance to veterinary patients is medication history. More than 60 drugs have been reported to induce BP, including some antibiotics, diuretics, antihypertensives, anti-TNF-a drugs, and vaccines. However, one of the highest correlations between prior use and BP is the drug class of dipeptidylpeptidase-4 inhibitors, used for the treatment of type II diabetes mellitus. While developed for their ability to degrade glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), it also has the additional ability to cleave N-terminal dipeptides on molecules like eotaxin (CCL11), RANTES, CCL5, CXCL9, CXCL10, and CXCL11. With veterinary medications typically lagging human development, we may someday see medications like sitagliptin, alogliptin, saxagliptin, or linagliptin.2,6
While a variety of inflammatory cells are found around the sites of BP, recent research has renewed focus on the role of mast cells in the development of this disease. More than 40 years ago, it was noted that an increased number of mast cells and increased degranulation of mast cells were present in the earliest stages of these lesions. Using tryptase as a marker for mast cells, research has shown increased tryptase levels in BP blister fluid, suggesting an early role for mast cells. While BPA2 IgG autoantibodies make up most of the antibody response, IgE autoantibodies with the same or similar epitope specificity are present in 70-90% of human lesions. Patients treated with omalizumab, a monoclonal antibody designed to inhibit IgE binding to FceRI, experienced decreased disease severity.1 Additional research with a focus on veterinary patients may reveal similar findings.
References:
- Fang H, Zhang Y, Li N, Wang G, Liu Z. The autoimmune skin disease bullous pemphigoid: the role of mast cells in autoantibody-induced tissue injury. Frontiers in Immunology. 2018;9:407.
- Garcia-Diez I, Ivars-Lleo M, Lopez-Aventin D, et al. Bullous pemphigoid induced by dipeptidyl peptidase-4 inhibitors. Eight cases with clinical and immunological characterization. International Journal of Dermatology. 2018;57(7):810-816.
- Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Saunders Elsevier; 2016: 602-604.
- Olivry T. Auto-immune skin diseases in animals: time to reclassify and review after 40 years. BMC Veterinary Research. 2018; 14:157.
- Scott DW. Pemphigus vegetans in a dog. Cornell Vet. 1977; 67(3): 374-84.
- Sundburg CR, Belanger JM, Bannasch DL, et al. Gonadectomy effects on the risk of immune disorders in the dog: a retrospective study. BMC Vet Res, 2016; 12(1): 278.
- Tasanen K, Varpuluoma O, Nishie W. Dipeptidyl Peptidase-4 Inhibitor-Associated Bullous Pemphigoid. Frontiers in Immunology. 2019;10:1238.
- Vassileva S, Drenovska K, Manuelyan K. Autoimmune blistering dermatoses as systemic diseases. Clin in Derm. 2014; 32(3): 364-375.