Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 23
April 25th, 2018
CASE II: UFMG 247/12 (JPC 4018791).
Signalment: 9-month-old, male, mixed breed (Felis catus), feline.
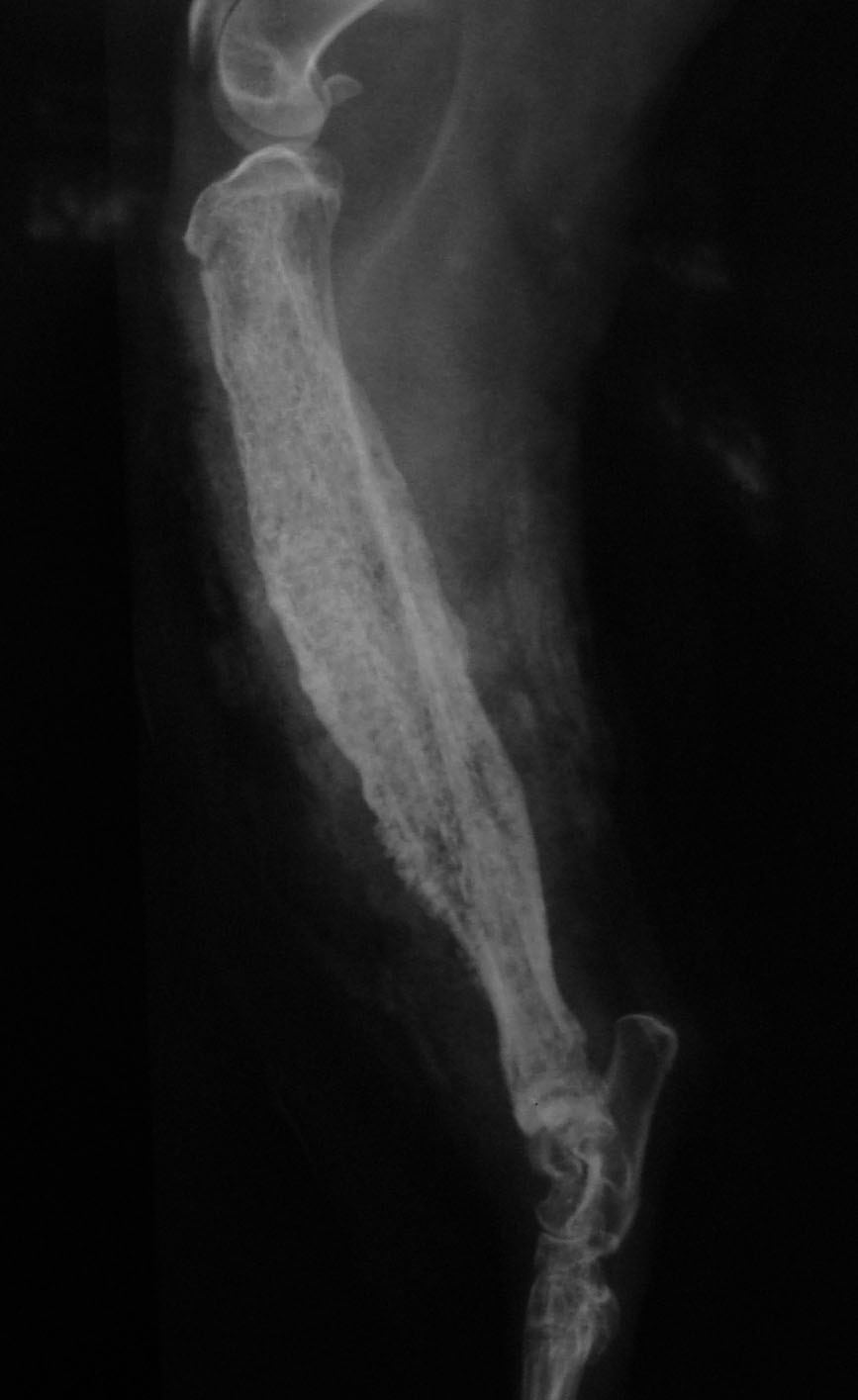
History: Initially this cat had a history of lameness of the right hind limb. The owner reported that the lameness started after a surgical procedure (orchiectomy) five months ago. Some days later, the cat returned to the same clinic where the orchiectomy had been performed. The right hid limb of this animal was subjected to radiography (craniocaudal and mediocaudal), and periosteal thickening of the metaphysis of the tibia was observed. Prednisone and enrofloxacin were prescribed, but improvement was not observed. After 50 days, two draining fistulae were observed on the medial tibia and topic rifamycin was prescribed. Fifteen days later, more draining fistulae were detected, and treatment with prednisone and enrofloxacin was reinitiated. Due to poor response to therapy and progressive weight loss, the cat was submitted to the veterinary hospital at the Universidade Federal de Minas Gerais (UFMG). On the clinical examination, the cat had temperature of 39oC, regular body condition and the right tibia was diffusely thickned. Radiography was performed, and blood was collected for hemogram and biochemical analysis. Radiography revealed marked changes in the metaphysis and diaphysis of the right tibia. Changes were characterized by extensive loss of cortical bone and replacement by irregular and disorganized bone. There was no cortex-medullar definition and irregular radiodensity (osteoproliferation) was observed within marrow bone. In addition, marked periosteal reaction and thickening of the soft tissues lateral to the tibia were observed (Figure 1). Considering the presence of deep dermatitis, myositis, and involvement of the marrow bone (probably osteomyelitis), another therapy was attempted. A treatment with cefazolin, meloxicam, cefovecin sodium, tramadol, and fluid therapy was prescribed. After four days, a new blood test was performed, exudate was collected for bacteriological examination, and a surgical curettage was performed. The cat responded poorly to treatment, and a new blood test did not indicate any recovery. Due to unfavorable prognosis, amputation of the referred right hid limb was performed. One week after amputation, the clinical condition improved notably and no sign of disease was detected again.
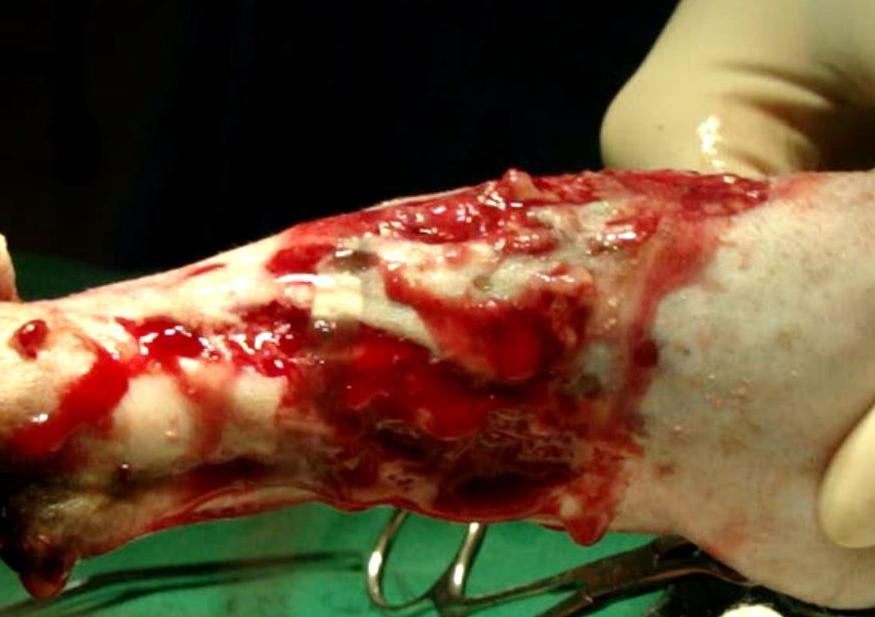
Gross Pathology: Grossly, the skin along right tibia was intensely and multifocally to coalescing ulcerated. A reddish-yellow viscous exudate was observed in the subcutis and muscles (Figure 2). The right tibia was enlarged with moderate new bone formation along the metaphysis and diaphysis. Longitudinal and transverse sections showed loss of the medullary cavity and replacement with white and firm woven bone with scattered yellowish-white and granular material. There was no evidence of differentiation between medullary cavity and cortical bone.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.):
Clinical pathology:
- CBC two weeks prior amputation: 4,6 Hgb, 17,0 Hct, 4,0 RBC, 86/34.744 Seg, 08/ 3232 Lym, 03/1212 Mno.
- CBC one day prior amputation: 6,7 Hgb, 24,0 Hct, 6,0 RBC, 90/28.630 Seg, 07/ 1.449 Lym, 01/207 Mno.
- No changes were detected in the biochemical profile.
Microbiology: Pseudomonas aeruginosa was isolated from draining purulent exudate.
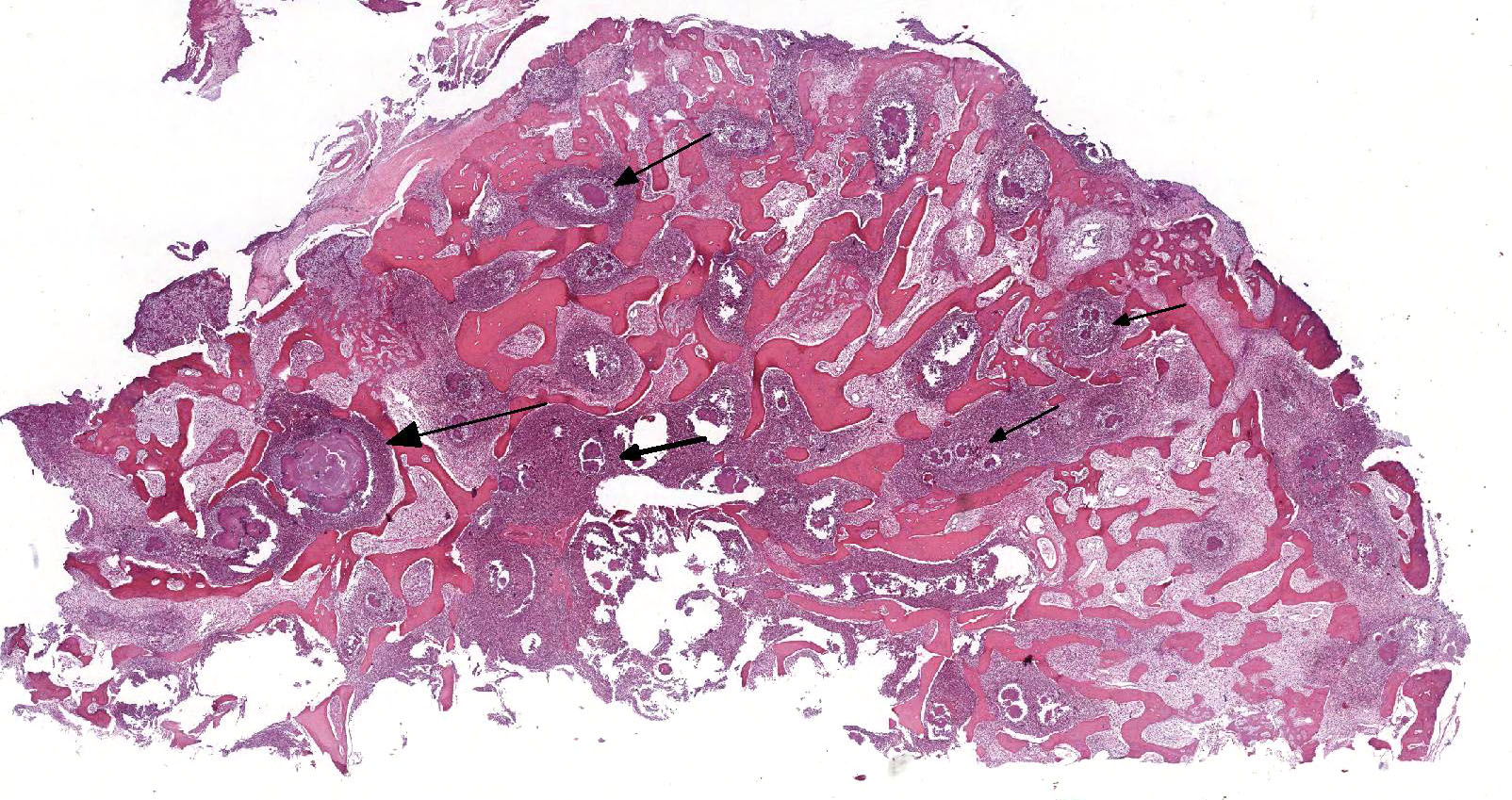
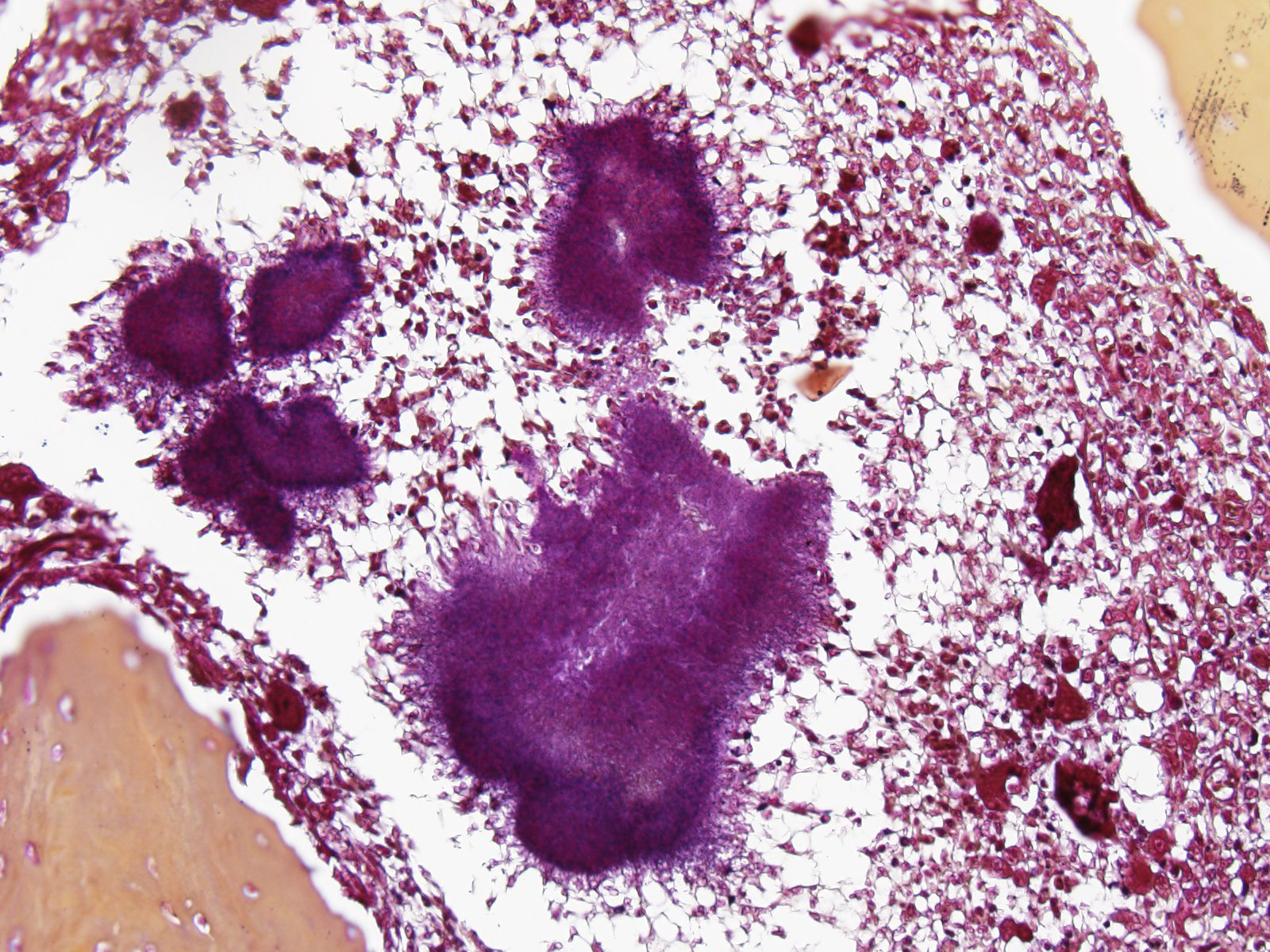
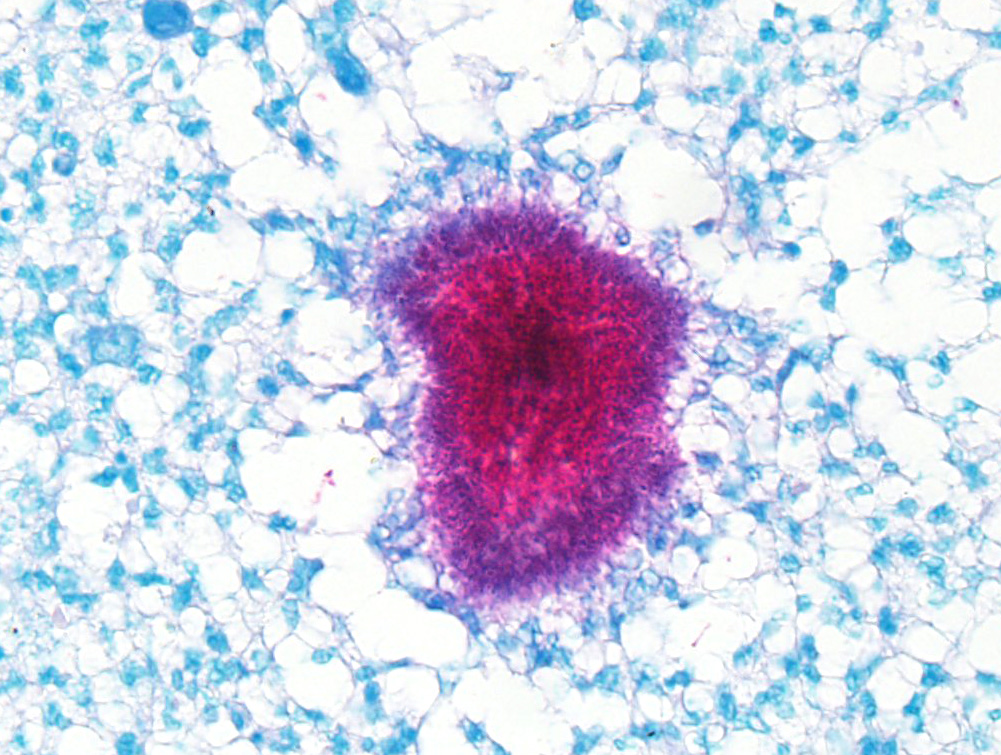
Microscopic Description: Histologically, the skin was extensively ulcerated and replaced by many neutrophils and cellular debris. In the subjacent dermis, there was multifocal mature granulation tissue and numerous neutrophils and macrophages. Deep in the dermis, multifocal variably-sized areas with colonies of bacteria surrounding by neutrophils, macrophages and giant multinucleated cells are found and irregular format with basophilic centers and eosinophilic filamentous radiating aggregates in the periphery (Splendore-Hoeppli phenomenon). Adjacent to the pyogranulomatous reaction, connective fibrous tissue and neovascularization were observed. The reaction invaded the hypodermis and muscular fascia. Where the infection reaches the muscles, marked necrosis and loss of muscular fibers could be seen. The periosteum of the tibia was thickened due to marked fibrous tissue proliferation and inflammation. The compact bone of cortical was multifocally replaced by an inflammatory reaction and trabecular bone. The Harvey’s canals were expanded and there was bone necrosis (loss of osteocytes) and reabsorption. The marrow bone was replaced by multiple Splendore-Hoppli reactions surrounding by neutrophils and giant cells (several containing 20 to 60 nuclei). Many foamy macrophages were infiltrating interconnected trabecular bone containing few osteoblast cells and no mineralized osteoid layer. Multifocal areas with new endosteal bone forming a network were found among remnants trabeculae. Thromboses were observed in the periosteal layer in some slides. Popliteal lymph node presented marked cortical lymphoid hyperplasia. Within lymphatic sinuses, numerous macrophages and giant cells with foamy cytoplasm were observed. Selected tissues from tibia, lymph node and adjacent muscles were subjected at Good Pasture, Giemsa, periodic acid – Schiff (PAS), Ziehl Neelsen and Grocott's methenamine silver (GMS) special stains. Good Pasture stained Gram-positive bacteria within colonies in the marrow bone, periosteum, muscles, fascia, skin and lymph node. A Goodpasture gram stain revealed many coccobacteria grouped in the central area or associated to the filamentous radially arranged material. In the periphery of reaction, cocci grouped in pairs or single chains could be seen. No organisms were identified in identical tissue sections stained by all others special stains aforementioned.
Contributor’s Morphologic Diagnosis:
- Bone (tibia): Marked diffuse pyogranulomatous periostitis, osteomyelitis, and marrow osteoproliferation associated with numerous granule formation (Splendore-Hoeppli phenomenon), and multifocal cortical loss, reabsorption, fibroplasia and new endosteal bone formation.
- Skin and muscles (not included): Marked multifocal to coalescing pyogranulomatous and necrotic dermatitis and myositis associated with numerous granule formation (Splendore-Hoeppli phenomenon)
Contributor’s Comment: Bacterial pseudomycetoma (botryomycosis) is a pyogranulomatous skin disease characterized by an unusual, presumed immunologic, reaction to nonfilamentous bacteria4. Some specific bacteria elicit Splendore-Hoeppli reaction (an antigen-antibody complex), which are characterized by the presence of radiating, club-shaped eosinophilic material around infectious and non-infectious agents6. Grossly, purulent material discharged from fistulae frequently contains white to yellow sand-like granules3. The antigen-antibody complex, morphologically unique reaction, was first described in sporotrichosis by Splendore and is schistosomiasis by Hoeppli6. Splendore-Hoeppli reaction can be caused by filamentous bacteria including Actinomyces spp. and Nocardia spp.4. Also, nonfilamentous bacteria including Pseudomonas, Proteus, Eschericiha coli6, Staphylococcus and Streptococcus4 are described and should be considered in the differential diagnosis. Gram’s stain for bacteria can be used for differential diagnosis among Gram-positive (Actinomyces, Nocardia, Staphylococcus, Streptococcus) and Gram-negative (Pseudomonas, Proteus and Eschericiha coli) organisms6. Also, Nocardia spp. can be identified by Ziehl Neelsen5, PAS and GMS stains.3
In cats, infectious with Actinomyces viscosus8 and Nocardia spp.5 were reported. However, report of nonfilamentous bacteria causing pyogranulomatous infection in the skin, muscles and bone in cats was not found.
The bacteria isolate in this case (Pseudomonas aeruginosa) could not be identified on special stains. Myriads of slightly elongate Gram–positive bacteria arranged in pairs or single chains were suggested of Streptococcus spp. infection. However, Staphylococcus spp. cannot be excluded. Gram-negative bacilli suggested of Pseudomonas spp. were not detected in all tissues evaluated.
Clinically, fungal organisms including Microsporum canis causing dermatophytic pseudomycetoma should be considered for differential diagnosis in cats. In fungal infection, the histopathology is very useful for differential diagnosis. Fungal hyphae are visible within granulomatous inflammation in tissue stained by hematoxilin and eosin and are strongly positive using PAS and GMS special stains.4
Skin and subcutaneous infections probably to develop as a result of wound contamination or trauma such as bites, lacerations, or puncture wounds with foreign bodies. Thus, infections localized in the skin and subcutis may extend deep to involve bone and muscle.3 Bacterial infectious of bones usually originates in vascular areas of periosteum (determining periostitis) or medullary cavity (determining osteomyelitis). During bacteremia or septicemia, bacteria can become localized in many organs. In bones, there is a strong predilection for sites of active endochondral ossification within the metaphyses and epiphyses of long bones and vertebral bodies. The medullary sinusoidal capillaries are fenestrated, permitting ready escape of bacteria into the bone marrow. Thus, a combination of physeal, metaphyseal, or epiphyseal injury and concurrent bacteremia may be involved in the pathogenesis of hematogenous osteomyelitis.10 Probably, extension of infection to periosteum and marrow bone from adjacent tissue (skin and muscles) occurred in this animal. Indeed, evidence of other foci of infection was not detected in this cat.
Conversely, bacteria can be localized in the bone marrow and the infection can disseminate to adjacent tissues. Clinical manifestations of osteomyelitis may not develop until several months later when the bone lesion becomes extensive enough to cause pain, disfigurement of the bone, or perhaps result in pathological fracture.10
The recognition of reactive conditions associated with Splendore-Hoeppli reactions and differentiation among different agents (bacteria or fungi) are important to therapeutic measures. Also, aseptic collection of exudate and confidence culture following antibiogram is important to obtain success in the treatment.
JPC Diagnosis: Bone: Osteomyelitis, pyogranulomatous, chronic-active with diffuse, severe osteolysis and multifocal woven bone production with numerous colonies of filamentous bacilli, mixed breed (Felis catus), feline.
Conference Comment: Osteomyelitis, or inflammation of the medullary cavity and adjacent bone, is most commonly caused by bacteria (rather than fungi). Bacteria can spread to bone by three routes: (1) hematogenously, (2) local extension, or (3) implantation.1
Hematogenous spread is most common in young horses and ruminants with omphalophlebitis, however, these animals usually succumb to septicemia before boney lesions become evident. Bacteria seem to prefer areas of active endochondral ossification within metaphyses and epiphyses of long bones and vertebral bodies for two reasons, (1) capillaries there are fenestrated and allow bacteria to enter the bone marrow and (2) they make sharp turns within the growth plate allowing for accumulation of bacteria. Local extension is most common in older animals with severe periodontal disease and secondary bacterial spread into the maxilla and mandible, and implantation of bacteria usually results from adulteration of surgical fracture repair, bite wounds or gunshot injury, or open fractures.1
Certain bacteria are partial to bone, like Staphylococcus aureus, which invades osteoblasts, protecting it from host immune defense.1 Pasteurella multocida wreaks havoc by retarding the growth and differentiation of osteoblasts and activating osteoblasts to resorption.2 Moreover, at sites of infection, host defense mechanisms can be counter-productive, for example, cytokine production (IL-1, IL-6, TNF-α) by inflammatory cells induces osteoblasts to activate osteoclasts to resorb bone.1 A summary of the numerous manifestations of bacterial osteomyelitis is below for your viewing pleasure.
Table 1: Common manifestations of bacterial osteomyelitis1
|
Location |
Species affected |
Predisposing factor |
Route of dissemination |
Common isolates |
|||
|
Vertebral body |
Horses, livestock |
Inadequate passive immunity |
Hematogenous (through umbilicus) |
Trueperella pyogenes (common) Foals: Escherichia coli, Salmonella enterica serovar Typhimurium, staphylococci, streptococci, Rhodococcus equi Calves: Fusobacterium necrophorum Sheep: Mannheimia haemolytica, F. necrophorum, staphylococci Pigs: Erysipelothrix rhusiopathiae, staphylococci, streptococci |
|||
|
Piglets, lambs |
Inadequate passive immunity |
Hematogenous (tail biting or docking) |
|||||
|
Mandible |
Cattle “lumpy jaw” |
Penetrating injury to oral mucosa |
Implantation |
Actinomyces bovis or Trueperella pyogenes |
|||
|
Numerous |
Periodontitis |
Local extension |
Fusobacterium necrophorum, other oral commensals |
||||
|
Numerous locations |
Small animals |
Open fractures, bite wounds, gunshot injury |
Implantation |
Staphylococcus pseudointermedius, streptococci |
|||
|
Nasal cavity |
Pigs |
Bacterial toxins |
Local extension |
Pasteurella multocida and Bordetella bronchiseptica |
|||
Pseudomonas aeruginosa, isolated from the purulent exudate in this case, is traditionally thought of as the causative agent of numerous dermatologic disorders, such as post-grooming furunculosis and otitis externa.9 However, as a potent gram-negative bacterium, it can cause a myriad of clinical syndromes (endometritis (mares), mastitis (cattle), pneumonia (foals and mink), enteritis (primates), and abortion and abnormal fetal development (cattle and horses)) and, in these cases, is often part of a consortium of fellow gram-negative and gram-positive bacteria.7
Ruleouts discussed in this case included: Nocardia sp. and Actinomyces sp. The moderator and conference attendees noted that they didn’t appreciate Splendore-Hoeppli material (radiating eosinophilic club-shaped antigen-antibody protein) surrounding bacterial colonies. Gram and acid-fast stains were run to further classify the bacteria which in the submitted unstained sections appear to be gram-positive and acid-fast. In the moderator’s and attendee;s cumulative experience, these organisms are most indicative of Nocardia sp, which we favor as the causative agent in this case.
Actinomyces sp. and Nocardia sp., often lumped together in textbooks, come in cutaneous, subcutaneous, and visceral forms. They generally manifest as nodular ulcerated lesions on the extremities which may arise from underlying bone and are often secondary to wound contamination. Grossly, yellow to green sulfur granules are present corresponding with microscopic aggregates of bacteria with small amounts of adherent neutrophils awash in a sea of suppurative or pyogranulomatous inflammation. Attendees agreed that the aggregates of fulamentous bacilli in these sections are strongly reminiscent of sulfur granules.
Contributing Institution:
Universidade Federal de Minas Gerais
Escola de Veterinária
Departamento de Clínica e Cirurgia Veterinárias
Av. Antônio Carlos, 6627; 31270-901.
Belo Horizonte, MG, Brazil
www.vet.ufmg.br
References:
- Craig LE, Dittmer KE, Thompson KG. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, Vol 2, 6th ed. St. Louis, MO: Elsevier; 2016:98-103.
- Gwaltney SM, Galvin RJ, Register KB, Rimler RB, Ackermann MR. Effects of Pasteurella multocida toxin on porcine bone marrow cell differentiation into osteoclasts and osteoblasts. Vet Pathol. 1997; 34(5):421-430.
- Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Jubb, Kennedy & Palmer's Pathology of Domestic Animals, Maxie MG, 5th ed., pp. 553-781, Saunders Elsevier, Toronto, Canada, 2007.
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin diseases of the dog and cat: clinical and histopathological diagnosis, second ed., p. 01 – 932, Blackwell Science, Oxford, United Kingdom, 2005.
- Harada H, Endo Y, Sekiguchi M, Setoguchi A, Momoi Y. Cutaneous nocardiosis in a cat. J Vet Med Sci, 71:785-787, 2009.
- Hussein MR. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol, 35: 979-988, 2008.
- Kahn CM. The Merck Veterinary Manual. 9th Whitehouse Station, NJ: Merck & Co., Inc.; 2005:422, 1132, 1544, 1550, 1753.
- Murakami S, Yamanishi MW, Azuma R. Lymph node abscess due to Actinomyces viscosus in a cat. J Vet Med Sci 59:1079-1080, 1997.
- Tham HL, Jacob ME, Bizikova P. Molecular confirmation of shampoo as the putative source of Pseudomonas aeruginosa-induced postgrooming furunculosis in a dog. Vet Dermatol. 2016; 27(4):320-380.
- Thompson K: Bones and joints. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Maxie MG, 5th ed., pp. 02-184, Saunders Elsevier, Toronto, Canada, 2007.