Signalment:
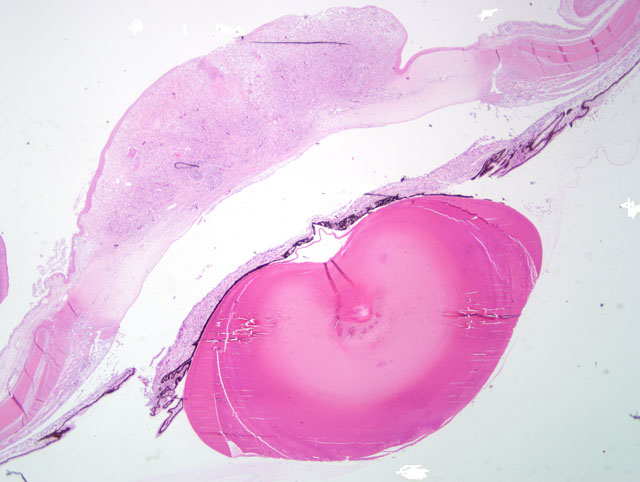
Gross Description:
Histopathologic Description:
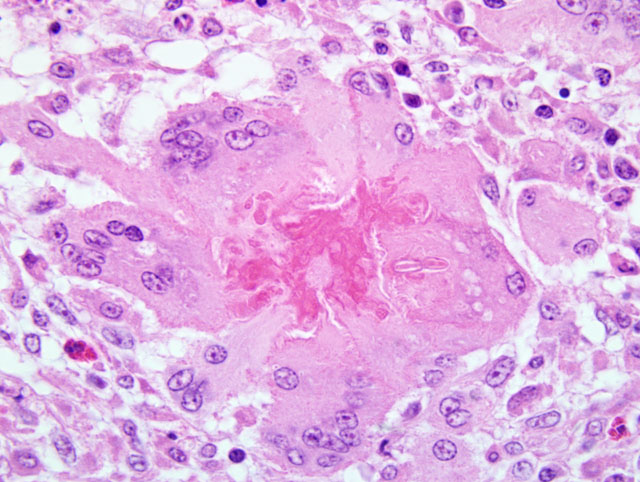
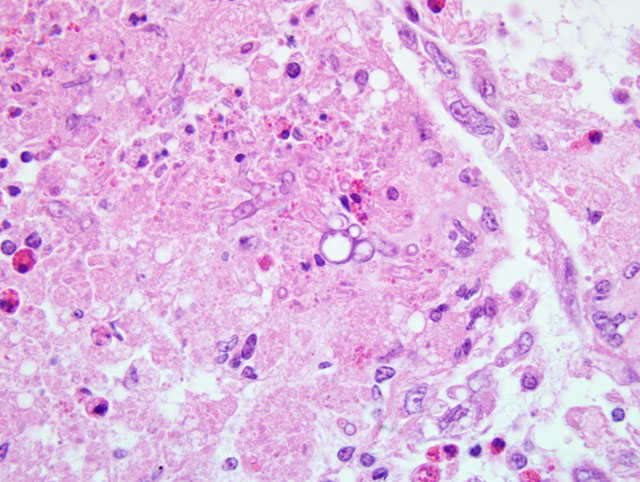
The stroma showed patchy to extensive necrosis, and activation and proliferation of keratocytes. Within the inflammation, particularly near areas of necrosis in the superficial and mid stroma there were few to moderate numbers of branched, septate fungal hyphae. Staining with both PAS and GMS highlights the fungi.
Endothelium and Descemet's membrane were missing from the central corneal region at the area of full thickness inflammation. There was a wedge of fibrous tissue bridging the gap. In the worst affected birds, Descemet's membrane, which is very thin in this age of bird, could not be detected. Where present, endothelial cell density was considered normal.
In birds with obliteration of the anterior chamber there is extensive adhesion of the iris to the posterior cornea, particularly at the areas of full thickness inflammation. In these birds the iris was only identifiable by the lines of pigment. In other birds there is moderate to severe infiltration of the iris and ciliary body with inflammatory cells of similar mixture to those in the cornea. Granulocytes predominate with few giant cells.
Lens epithelium was irregular and heaped up centrally and at one pole in more moderately affected birds. The worst affected birds had wrinkles and sometimes fragmented lens capsule outlining a pocket of proliferative epithelial cells. These birds had marked lymphocytic inflammation of the pecten but the posterior segment in all birds was otherwise unremarkable except for some retinal folding, which may be normal for this age.
There was moderate to severe lymphocytic conjunctivitis in some birds more evident ventrally, in the birds that had it, than in the dorsal lids. There was no evidence in any submitted eye of chlamydophilosis.
Morphologic Diagnosis:
Severe chronic diffuse, mixed cellular, mycotic keratitis.
Severe chronic active iritis with extensive anterior synechia.
Cataract and pectenitis secondary to intraocular inflammation.
Condition:
Contributor Comment:
Fungal lesions arising in the cornea may extend to involve the anterior segment, but rarely progress into the posterior part of the eye; in contrast, endophthalmitis, associated with fungal respiratory disease, typically affects the retina and vitreous, but the cornea remains unaffected. Interestingly, corneal infections are almost always unilateral.
In all previous reported cases of fungal keratitis in chickens, the isolated agent has been Aspergillus fumigatus which makes this outbreak unique. Scedosporium apiospermum (and its teleomorph, or sexual stage, Pseudallescheria boydii) is a filamentous fungus of widespread distribution, recently described as one of the clinically significant emerging mycoses.(17) It has, however, a long history, being first recognized in human otitis in the late 19th century, and in the mycetoma known as madura foot in the early 20th. Taxonomy has been confused, but the present designation is S. apiospermum for the asexual state and P.boydii for the sexual.
S. apiospermum is best known for its association with near drowning events and subsequent pneumonia and disseminated disease.(5,17) Its tolerance to cold, low oxygen tension and high salinity make it a survivor in polluted environments and recovery of the species from unpolluted environments is rare.(5) Cytological and histological distinction from Aspergillus sp is difficult. The two genera are closely related and share antigenic epitopes in formalin fixed tissues.(17)
Corneal infections have been reported in humans and are associated with a guarded prognosis for sight because of the resistance of this fungus to commonly used therapeutic agents.(16) To date no other species has been reported with this infection in the cornea or ocular tissues, but the increasing rate of environmental detection of S. apiospermum suggests that infection may become more common in domestic species and poultry as it has in humans.
JPC Diagnosis:
1. Eye: Keratitis, ulcerative, granulomatous and heterophilic, diffuse, severe with ulcerative conjunctivitis and fungal hyphae.
2. Eye, iris: Anterior uveitis, heterophilic and lymphoplasmacytic, diffuse, marked with anterior synechia.
3. Eye, lens: Cataract.
Conference Comment:
Most attendees identified the fungal hyphae as Aspergillus species. Given the morphologic similarity between S. apiospermum and Aspergillus sp., the confusion is understandable and highlights the utility of microbial culture in arriving at a definitive diagnosis when mycotic organisms are observed during histologic examination of tissues. Because mild conjunctivitis was present in some conference attendees slides, participants briefly reviewed several common causes of conjunctivitis in avian species, among which include: Chlamyadia psittaci, Newcastle disease virus, avian influenza virus, infectious bronchitis virus, infectious laryngotracheitis virus, and fowl poxvirus. Likewise, causes of cataract formation in the bird eye were discussed, two of which include: vitamin E deficiency and avian encephalomyelitis virus.(14) Additionally, congenital cataracts of unknown etiology sporadically occur in commercial turkeys.(14)
The contributor provides detailed information on S. apiospermum as an important emerging mycotic disease. The moderator stressed that, in addition to the outbreak of S. apiospermum in this flock of chickens, S. apiospermum (Pseudallescheria boydii) has been noted to occur in elephant seals(8) and in the nasal passages of cattle,(13) horses,(6) and dogs.(2,3,4) In addition to the nasal passage, bones and joints appear to be other common locations for infection in dogs.(7,9) Other species of Scedosporium have been reported to cause osteomyelitis in animals, including S. prolificans in a horse(15) and S. inflatum in a dog.(12) In contrast to humans, S. apiospermum infection in animals has not yet been linked to immunosuppression.(13)
References:
2. Cabanes FJ, Roura X, Garcia F, Domingo M, Abarca ML, Pastor J. Nasal granuloma caused by Scedosporium apiospermum in a dog. J Clin Microbiol. 1998;36:2755-2758.
3. Caro-Vadillo A, Garcia-Real I, Paya-Vicens MJ, Sainz-Rodriguez A, Rodriguez-Franco F, Rodriguez-Bertos A. Fungal rhinitis caused by Scedosporium apiospermum in laborador retriever. Vet Rec. 2005;157:175-177.
4. Coleman MG, Robson MC. Nasal infection with Scedosporium apiospermum in a dog. N Z Vet J. 2005;53:81-83.
5. Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21:157-197.
6. Davis PR, Meyer GA, Hanson RR, Stringfellow JS. Pseudallescheria boydii infection of the nasal cavity of a horse. J Am Vet Med Assoc. 2000;5:707-709.
7. Elad D, Perl S, Yamin G, Blum S, David D. Disseminated pseudallescheriosis in a dog. Med Mycol. 2010;48(4): 635-638.
8. Haulena M, Buckles E, Gulland FM, et al. Systemic mycosis caused by Scedosporium apiospermum in a stranded northern elephant seal (Mirounga angustirostris) undergoing rehabilitation. J Zoo Wildl Med. 2002;33:166-171.
9. Hugnet C, Marrou B, Dally C, Guillot J. Osteomyelitis and discospondylitis due to Scedosporium apiospermum in a dog. J Vet Diagn Invest. 2009;21:120-123.
10.Itakura C, Goto M, Fujiwara M. Pathological observation of fungal (Aspergillus fumigatus) ophthalmitis in chicks. Nippon Juigaku Zasshi. 1973;35:473-479.
11.Mustaffa-Babjee A. Specific and non specific conditions affecting avian eyes. Vet Bull. 1969;39:681- 687.
12.Salkin IF, Cooper CR, Bartges JW, Kemna ME, Rinaldi MG. Scedosporium inflatum osteomyelitis in a dog. J Clin Microbiol. 1992;30:2797-2800.
13.Singh K, Boileau MJ, Streeter RN, Welsh RD, Meier WA, Ritchey JW. Granulomatous and eosinophilic rhinitis in a cow caused by Pseudallescheria boydii species complex (Anamorph Scedosporium apiospermum). Vet Pathol. 2007;44:917-920.
14.Swayne DE. Eye and ear. In: ed. Riddell C, Avian Histopathology. 2nd ed. Tallahassee, FL: American Association of Avian Pathologists, Rose Printing; 1996:204-206.
15.Sweczek TW, Donahue JM, Hunt RJ. Scedosporium prolificans infection associated with arthritis and osteomyelitis in a horse. J Am Vet Med Assoc. 2001;218:1800-1802.