Signalment:
2 to 3-year-old male cynomolgus macaque (
Macaca fascicularis).This monkey was part of a study investigating candidate renal biomarkers. It received seven daily IV doses of two potential renal toxicants, everninomicin (30 mg/kg) and gentamicin (10 mg/kg). The study included pre- and post-dose monitoring of serum and urine chemistry with necropsy and histologic examination. Examined tissues included adrenal glands, kidneys, testes, heart, liver, skeletal muscle and urinary bladder at the end of a one week dosing period. Gentamicin is an aminoglycoside antibiotic for the treatment of gram negative bacterial infection that can induce proximal tubular necrosis.(2,5) Everninomicin is an experimental oligosaccharide antibiotic that has a renal toxicity profile that is similar to gentamicin.(3)
Gross Description:
None.
Histopathologic Description:
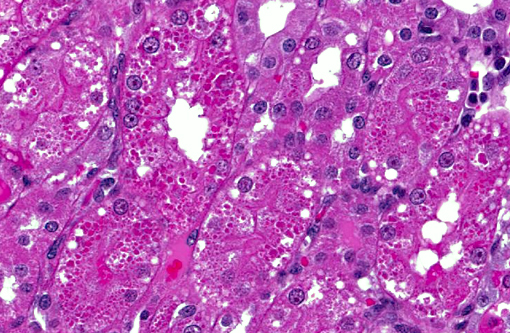
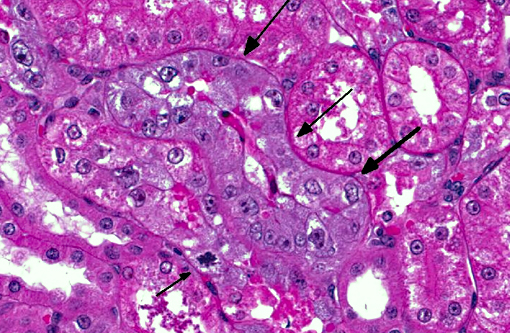
There were everninomicin- and gentamicin-related kidney histologic changes that correlated with serum and urine chemistry changes. There were minimal casts, mild regeneration and marked degeneration. Degeneration was characterized by proximal tubule epithelial cell swelling, cytoplasmic hyaline droplets, epithelial sloughing and epithelial cell necrosis. Regeneration included tubule epithelial cell cytoplasmic basophilia, crowding and slight increases in mitotic figures. There was also tubular dilatation with occasional scattered mononuclear cell accumulations in interstitium adjacent to degeneration or regeneration in some but not all sections.
Morphologic Diagnosis:
Kidney: Subacute diffuse renal proximal tubular degeneration and necrosis with regeneration.Â
Lab Results:
Serum and urine chemistry were tested twice prior to dosing (6 and 1 day prior to dosing) and 3 times during the week of dosing (post-dose days 3, 6 and 8). Values for the five time points are below. Standard units of measure and abbreviations were used unless otherwise noted. NS = not significant.Â
Clinical Chemistry:
| Parameter | Values |
| BUN | 125, 118, 101, 123, 89 |
| CREA | 125, 118, 101, 123, 90 |
| ALT | 125, 118, 101, 123, 91 |
| AST | 125, 118, 101, 123, 92 |
| AP | 125, 118, 101, 123, 93 |
| GGT | 125, 118, 101, 123, 94 |
| T BILI | 125, 118, 101, 123, 95 |
| TP | 125, 118, 101, 123, 96 |
| ALB | 125, 118, 101, 123, 97 |
| GLOB | 125, 118, 101, 123, 98 |
| A/G Ratio | 125, 118, 101, 123, 99 |
| CHOL | 125, 118, 101, 123, 100 |
| TRIG | 41, 31, 41, 41, 50 |
| Ca | 9.7, 9.7, 10.1, 10.0, 9.2 |
| PHOS | 6.8, 6.2, 6.9, 4.3, 4.6 |
| Na | 141, 143, 142, 141, 137 |
| K | 4.4, 4.3, 4.5, 3.4, 3.1 |
| Cl | 107, 107, 106, 110, 111 |
Urinalysis:
| Parameter | Values |
| pH | 8.5, 8.5, 8., 8.5, 7.5 |
| Protein | neg, neg, neg, trace, 3+ |
| Glucose | neg, 1+, 2+, NS, NS |
| Ketones | 1+, neg, neg, trace, 2+ |
| Bilirubin | neg, neg, neg, neg, neg |
| Blood | neg, neg, 1+, neg, neg |
| Specific Gravity | 1.019, 1.018, 1.018, 1.015, 1.017 |
| CREA | 103.6, 104.3, 87.6, 52.0, 67.1 |
| N-acetyl glucosaminidase (NAG) | 7.7, 7.2, 23.3, 28.0, 30.4 |
| Microalbumin | NS, NS, 7.2, 103.6, 143.3 |
| Total urine volume (ml/day) | 71.0, 65.0, 81.0, 127.0 |
Condition:
Aminoglycoside nephrosis
Contributor Comment:
Aminoglycosides cause acute renal failure by accumulating in proximal tubular epithelial cells where they damage mitochondria, ribosomes, and other intracellular components.(3) Proximal tubules resorb sodium, chloride, calcium, glucose, amino acids from glomerular ultra filtrate.(3,8) Insufficient resorption following loss of proximal tubular epithelium can cause hyperkalemia, sodium alterations, hyperphosphatemia, azotemia, acid-base disturbances, enzymuria, cylinduria oliguria, isosthenuria and/or anuria.(3) A concise yet comprehensive review of renal physiology and antibiotic-induced renal failure can be found at
http://www.vet.uga.edu/VPP/clerk/Matthews/index.php(5)
JPC Diagnosis:
Kidney, proximal convoluted tubules: Degeneration, necrosis and regeneration, diffuse, marked, with numerous cytoplasmic protein droplets.
Conference Comment:
The primary function of the renal glomerulus is blood filtration, while the proximal convoluted tubules are predominantly involved with absorption; in fact, sixty percent of all reabsorption of the filtered solute occurs in the proximal tubules. They are responsible for reabsorbing water, glucose, sodium, chloride, amino acids and calcium from glomerular ultrafiltrate; thus damage can result in the clinicopathologic findings enumerated above.(3) There are two main types of acute tubular necrosis: nephrotoxic and ischemic. Antimicrobials such as aminoglycosides (considered obligate nephrotoxins), heavy metals, chemotherapeutic agents and other nephrotoxins damage the tubular epithelium while sparing the basement membrane.(6) In contrast, renal ischemia is usually a consequence of hypotension; it is characterized histologically by tubular epithelial necrosis or apoptosis with disruption of the basement membrane.(6) In this case, the presence of an intact tubular basement membrane may be demonstrated with the periodic acid-Schiff stain, supporting a toxic etiology rather than an ischemic insult.
Considerable discussion surrounded the pathogenesis of aminoglycoside nephrotoxicity in animals. Common aminoglycosides employed in veterinary medicine (in decreasing order of nephrotoxicity) include neomycin, kanamycin, gentamicin, streptomycin, tobramycin and amikacin. Foals are particularly susceptible to aminoglycoside-induced nephrotoxicosis, while in cats, this class of drugs has been associated with ototoxicity as well as nephrotoxicity. Aminoglycosides typically accumulate within proximal tubular epithelial cell lysosomes and damage is thought to be dose-dependent. They induce tubular damage via a number of ways: destruction of the sodium-potassium pump with subsequent oncotic necrosis; inhibition of phospholipase with accumulation of lysomosmes filled with cellular membranes (myeloid bodies) impairment of mitochondrial respiration and cation transport; inhibition of protein synthesis; and loss of the epithelial brush border.(4,6,7)
In addition to the histological features described by the contributor, some conference participants noted that epithelial cells lining medullary collecting tubules are occasionally multinucleated, bulging into the tubular lumen. This is considered to be an incidental finding in cynomolgus macaques that is thought to be either a normal anatomic variation in this species or at best, a minor finding of little pathological significance.(1)
References:
1. Chamanza R, Marxfeld HA, Blanco AI, Naylor SW, Bradley AE. Incidences and range of spontaneous findings in control cynomolgus monkeys (Macaca fascicularis) used in toxicity studies. Toxicol Pathol. 2010;38(4): 642-657.
2. Davis JW , Goodsaid FM, Bral CM, et al. Quantitative gene expression analysis in a nonhuman primate model of antibiotic-induced nephrotoxicity. Toxicol Appl Pharmacol. 2004;200(1): 16-26.
3. DiBartola SP. Urinary system. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. Vol. 2. 7th ed. St. Louis, Missouri: Elsevier-Saunders; 2010:1976-1979.Â
4. Hottendorf GH, Williams PD. Aminoglycoside nephrotoxicity. Toxicol Pathol. 1986;14(1): 66-72.
5. Matthews C, Camus M, LeRoy B. Antibiotics and Acute Renal Failure. http://www.vet.uga.edu/VPP/clerk/Matthews/index.php
6. Maxie MG, Newman SJ. Urinary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Elsievier; 2007:466-470.
7. Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5): 1003-1012.
8. Plumb DC. Plumbs Veterinary Drug Handbook. 5th ed. Blackwell Publishing; 2005:206-209; 864-867.