Conference 22, Case 3
Signalment:
3-year-old, male, Merino X, Ovis aries, ovine
History:
Animal found dead two weeks after moved onto a paddock with a predominance of soursobs (Oxalis cernua) in pasture.
Gross Pathology:
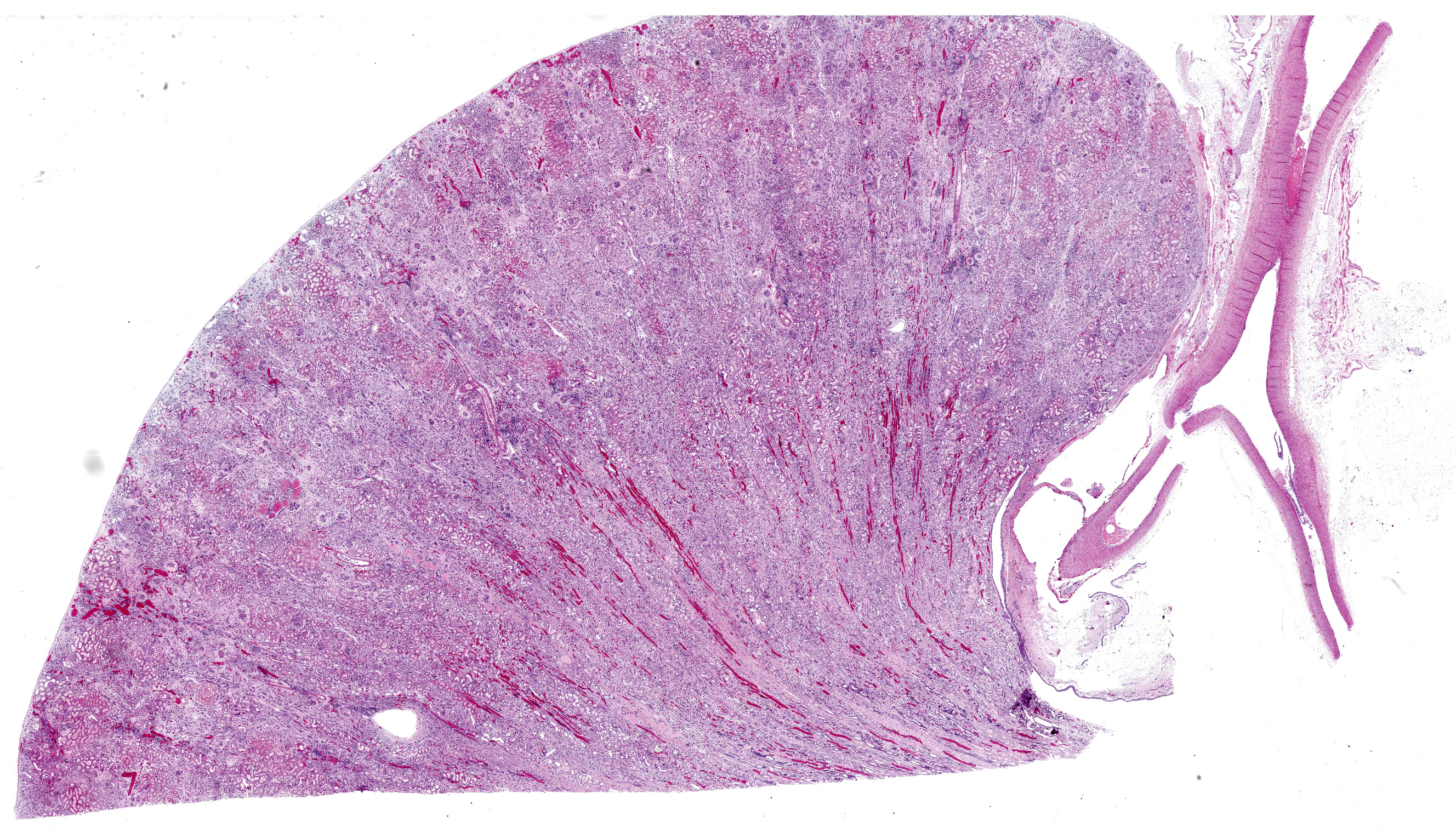
Post-mortem performed by referring veterinarian. Post mortem findings included heart petechial haemorrhages, leathery appearance of renal capsules and mild hepatomegaly.
Micrscopic Description:
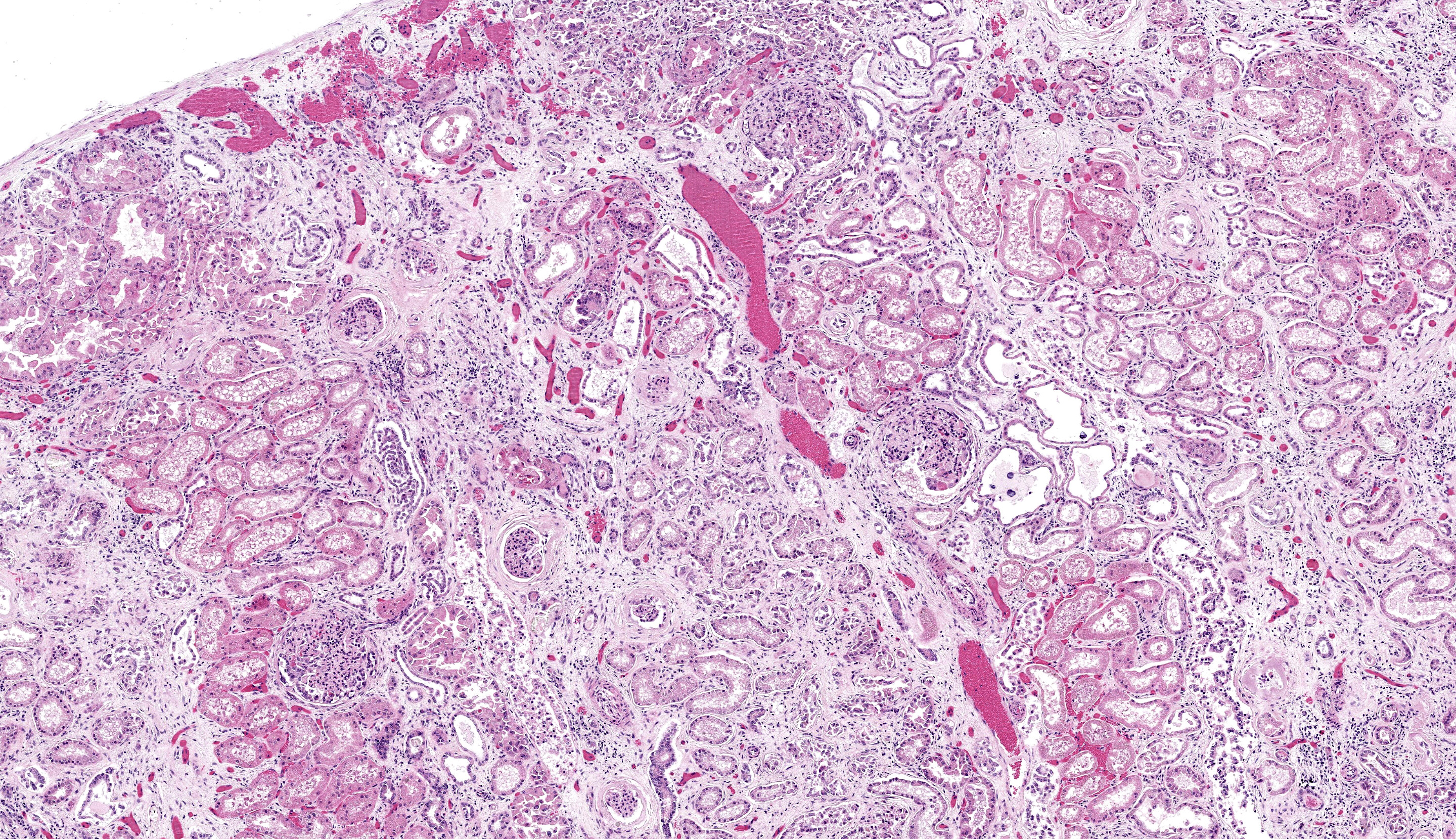
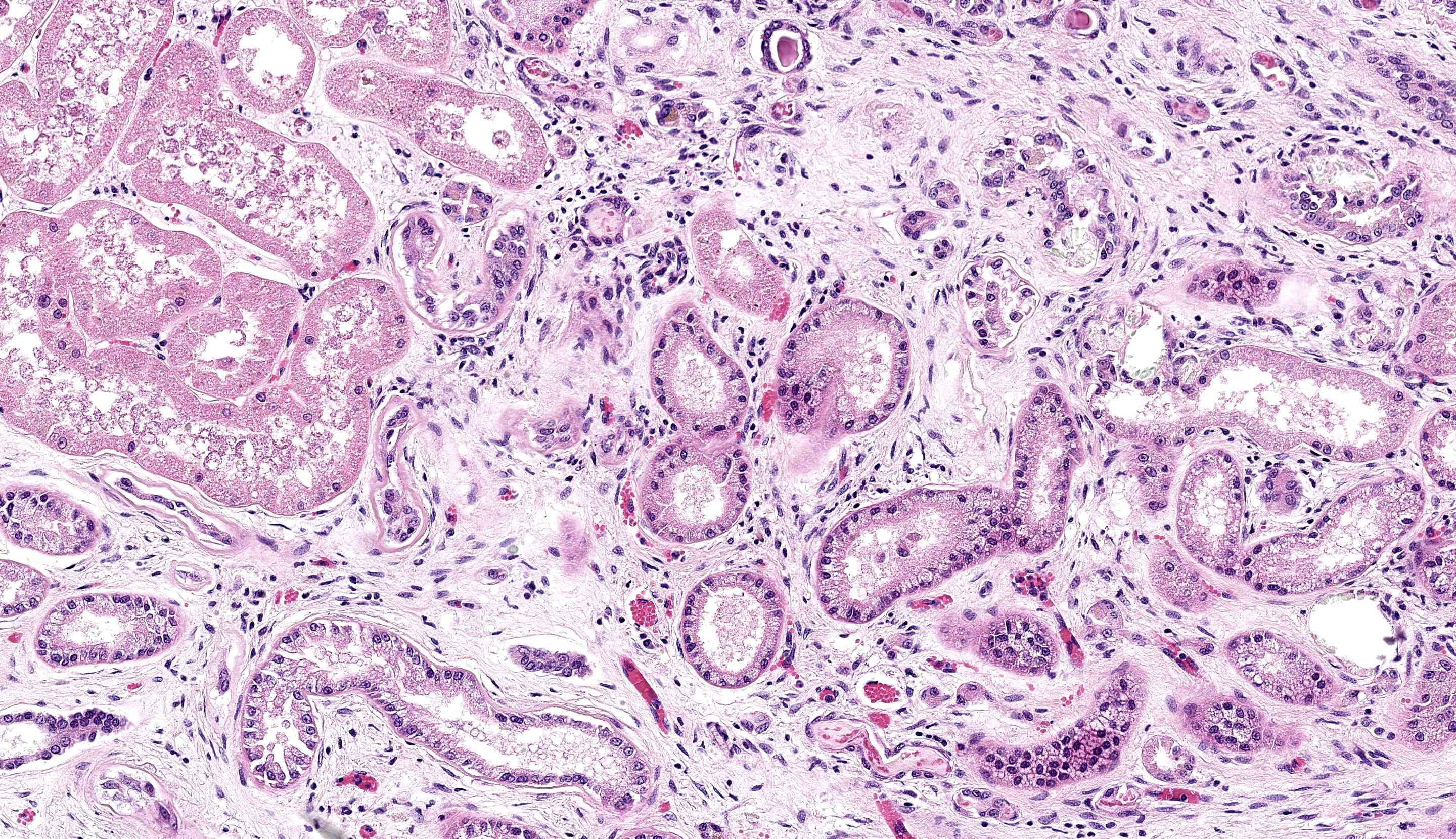
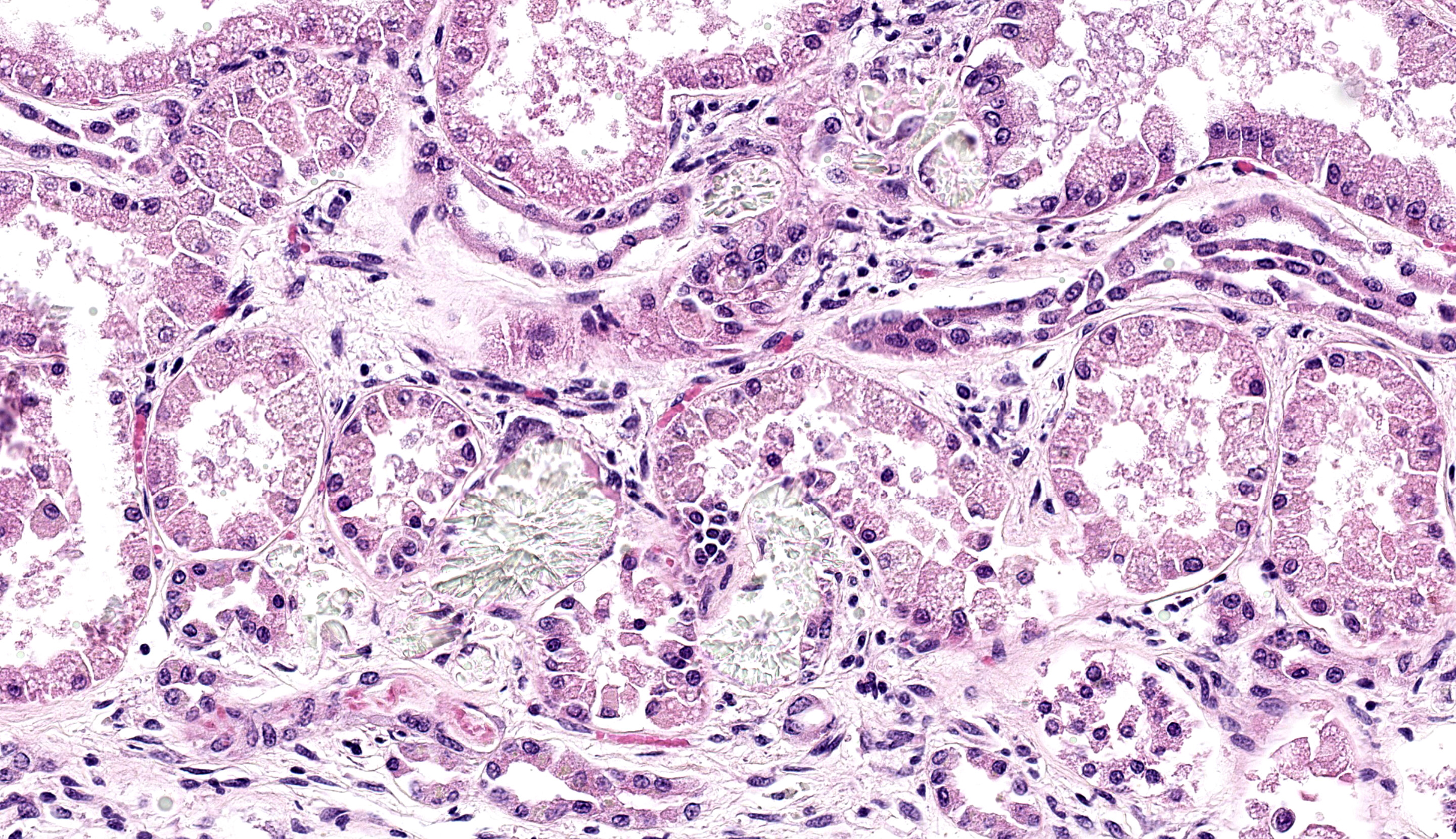
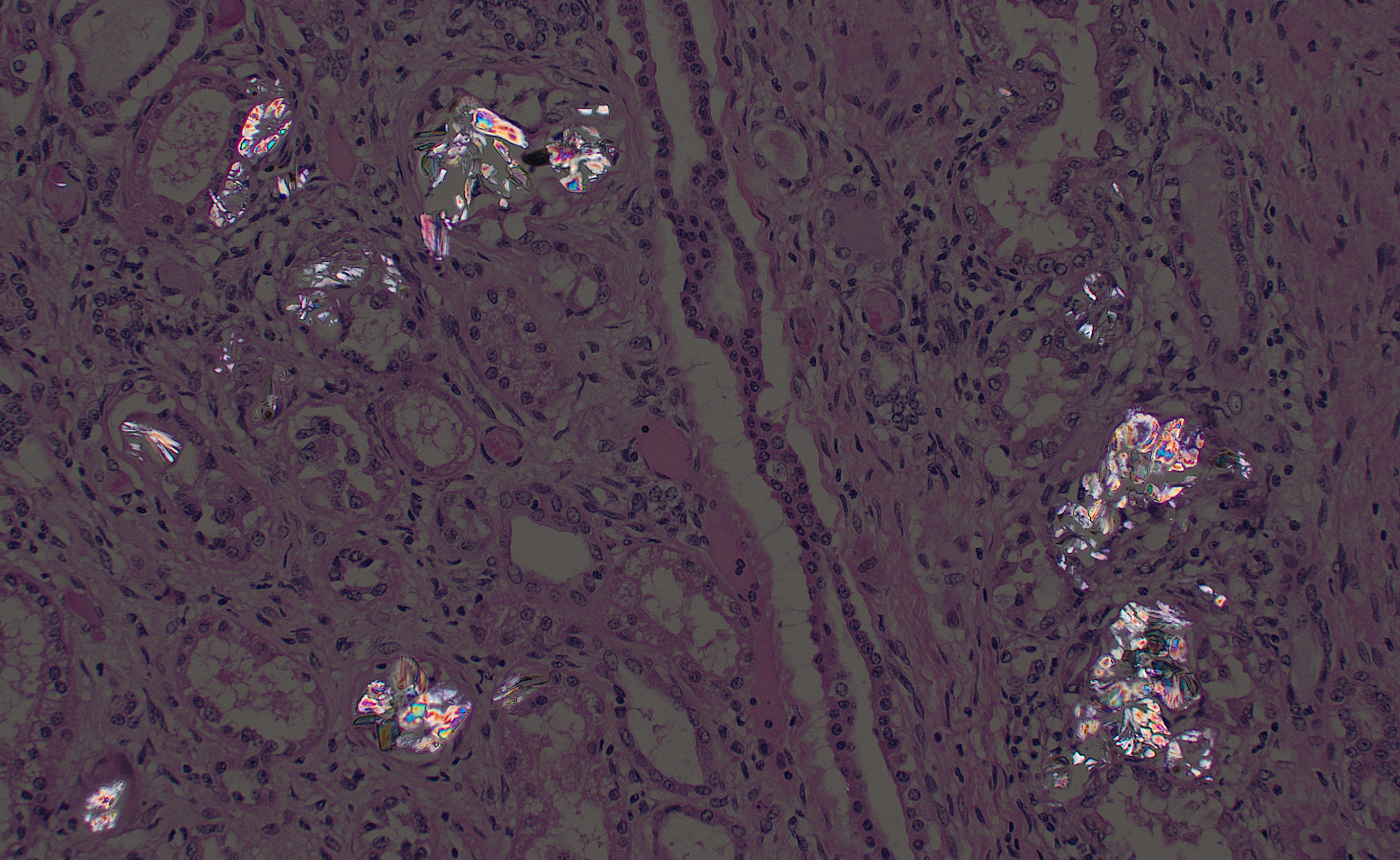
There is moderate multifocal predominantly cortical and less often medullary tubular loss, interstitial fibrosis and mild multifocal interstitial infiltrates of lymphocytes and plasma cells. Remnant tubules are often lined by degenerate, necrotic or attenuated epithelium. Bowman’s capsules and glomerular mesangium are thickened by fibrosis, with frequent synechia and scattered obsolescent glomerular tufts. Tubules are frequently ectatic, expanded up to 6 times normal diameter and filled with amorphous translucent eosinophilic material (protein), occasional sloughed epithelial cells and clear-yellow to lightly basophilic refractile crystals. Crystals are composed of radiating angular shards of variable size and shape and are birefringent under polarised light (calcium oxalate).
Contributor’s Morphologic Diagnosis:
Lymph nodes, spleen and liver: T-cell lymphoid hyperplasia, severe, diffuse
Liver: Pericholangitis and perivasculitis, mild, multifocal, lymphoplasmacytic with erythrophagocytosis and extramedullary hematopoiesis
Contributor’s Comment:
Oxalate nephrosis is the outcome of excessive calcium oxalate accumulation within renal tubules, forming insoluble crystals and resulting in tubular obstruction and acute renal failure.5 Tubular epithelial injury may also result from inflammation-mediated free-radical damage and interference with oxidative phosphorylation; however there is conjecture over the concentrations required to elicit these changes.5,12 Oxalate is the ionic form of oxalic acid and is derived from a number of sources.4 Endogenous production occurs in the liver via oxidation of glycolate to form glyoxalate or via the metabolism of hydroxyproline, a component of collagen. This is followed by conversion to oxalate by the action of lactate dehydrogenase.4 Primary hyperoxaluria (PH) is the predominant cause of oxalate toxicity in humans, caused by increased endogenous production due to defective enzyme activity.4 This may involve defects in alanine glyoxalate aminotransferase (PH1), glyoxalate/hydroxypyruvate reductase (PH2), or mitochondrial 4-hydroxy 2-oxoglutarate adolase (PH3).4 Primary hyperoxaluria is rare in domestic species, but has been reported in cats, dogs, and Beefmaster cattle, and is suspected in Australian wildlife species Gilbert’s potoroo (Potorous gilbertii) and koalas (Phascolarctos cinereus).1,5,6,13
Secondary oxaluria predominates in domestic and non-domestic species, caused by increased intestinal absorption or increased intake of oxalates or oxalate precursors such as ethylene glycol or ascorbic acid (vitamin C).1,4 In ruminants, oxalate toxicity is most commonly caused by ingestion of plants containing soluble oxalates, usually in the form of sodium or potassium oxalate. 3,5,10 This includes a vast range of plant species but is most commonly reported following ingestion of Halogeton glomeratus (halogeton), Sarcobatus vermiculatus (greasewood), Rheum rhaponticum (rhubarb), Oxalis spp. (soursobs) and Rumex spp. (sorrel, dock), Portulaca oleracea (purslane), Chenopodium album (lamb’s quarter), Bassia hyssopifolia (bassia), Amaranthus spp. (pigweed), Salsola tragus (Russian thistle) and Beta vulgaris (sugar beets).3,5,10 Oxalate concentration in these plants is dependent on a number of factors and varies between species. Higher concentrations are found in leaves rather than stems and seeds, and young plants commonly have higher concentrations than aged plants.5,10 Concentration is also known to decrease as the plant dries; however, plants with extremely high concentrations may be potent enough to cause intoxication even after a dry summer.5,10
After ingestion, soluble oxalates are rapidly absorbed from the gastrointestinal tract, resulting in acute disease as soon as 2 hours post-ingestion.9 In plasma, oxalates complex with calcium, causing hypocalcemia and precipitation of calcium oxalate within renal tubules and the vascular lumens of various tissues, resulting in vascular necrosis, haemorrhage and acute renal failure.5,10 The kidneys are the most severely affected due to their primary role in oxalate excretion.9 Ruminants are more tolerant of ingested oxalates than monogastrics due to the action of ruminal microbes such as Oxalobacter formigenes, which degrade oxalate to produce carbon dioxide and formate.5,11
Clinical signs of acute toxicity are caused largely by hypocalcemia and include ruminal stasis and subsequent bloat; twitching and tetany which may progress to seizures; and weakness and bradycardia.9 Animals that survive acute hypocalcemia may succumb within a number of days to acute renal failure, characterised by anorexia, depression, weight loss and diarrhoea.9 Sheep are more commonly affected than cattle, however both species are equally susceptible under experimental conditions, suggesting that differing grazing patterns may contribute to differences in natural susceptibility.5 The characteristic clinical and pathological findings of acute oxalate intoxication in sheep include hypocalcemia, azotemia, and nephrosis associated with precipitation of birefringent calcium oxalate crystals in renal tubules.3,7,8
Ruminal oxalate-degrading bacteria increase with gradual exposure to higher concentrations of oxalate, allowing adapted animals to consume greater quantities.2,3,11 The development of acute toxicity also varies depending on rate of consumption, the availability of water and other feed and the total amount consumed.10 Therefore, management relies on limiting availability of oxalate-containing plants and providing access to other feed sources.9
Contributing Institution:
Veterinary Diagnostic Laboratory, School of Animal and Veterinary Sciences, University of Adelaide Roseworthy Campus, Mudla Wirra Rd, Roseworthy SA, 5371, Australia
https://www.adelaide.edu.au/vet/about-us/pathology
JPC Diagnosis:
- Kidney: Tubular degeneration, necrosis, and loss, diffuse, with proteinosis, tubulorrhexis, and numerous oxalate crystals.
2. Kidney: Nephritis, interstitial, lymphoplasmacytic, chronic, diffuse, moderate, with glomerular synechiae and periglomerular and interstitial fibrosis
JPC Comment:
This third case prompted intense discussion among participants. The contributor provides a outstanding histologic section to accompany a quality summary of oxalate toxicosis. We homed in on the disparity between acute and chronic changes on the slide, aided by Masson’s trichrome, Jones methenamine silver, and PAS stains. We agree that the tubular changes, tubulorrhexis and leakage of the urinary filtrate into the interstitium, where it mimics edema fluid) reflect a primary process of a subacute time course, though we could not agree that the chronic interstitial nephritis followed from this same tubular obstruction. Masson’s trichrome showed marked interstitial and periglomerular fibrosis that does not fit with the relatively short time course between introduction to pasture and death of this animal. To satisfy these concepts for this case, we created two separate morphologic diagnoses and interpreted this case as superimposed acute renal failure on a background of chronic subclinical renal insult.
Some participants also commented on the possibility of calcium carbonate uroliths given that the morphology of some crystals did not completely fit with oxalates. Calcium carbonate uroliths are also common in ruminants, particularly when calcium availability is high. Habituation of Oxalobacter in the rumen to oxalate-containing forage presents one such opportunity as breakdown of calcium oxalate increases free calcium significantly.11 It is also worth noting that oxalate crystals can also be a normal finding in the urine of ruminants, especially when concentrated.11 In the present case, there is clear association with tubular changes to include tubulorrhexis that is outlined clearly with JMS and PAS stains.
There are other considerations for this case that were briefly entertained by the contributor already. Primary hyperoxaluria has been described in sheep breeds, to include the Zwartbles (a Dutch breed).14 The condition is autosomal recessive and involves a loss of function missense mutation in the gene encoding for alanine-glyoxylate aminotransferase (Type 1 hyperoxaluria). The associated nephropathy is severe and heterozygous sheep may still develop histologic changes within the kidney.14 That this animal was cross bred raises the possibility of a similar gene interaction. Finally, production of oxalates by fungi such as Aspergillus remain an uncommon possibility. Nephrosis following ingestion of contaminated feed has been previously described.15 It is possible that this case reflects a multifactorial pathogenesis that leaves conference participants satisfied not matter their prevailing theory of the case.
References:
- Diseases of the Urinary System. In: Veterinary Medicine, eds. Constable PD, Hinchcliff KW, Done SH, Grünberg W, 11 ed., pp. 1095-1154. Elsevier Ltd., 2017
- Allison M, Littledike E, James L: Changes in ruminal oxalate degradation rates associated with adaptation to oxalate ingestion. Journal of animal science 45: 1173-1179, 1977.
- Aslani MR, Movassaghi AR, Najarnezhad V, Pirouz HJ, Bami MH: Acute oxalate intoxication associated to ingestion of eshnan (Seidlitzia rosmarinus) in sheep. Trop Anim Health Prod 43: 1065-1068, 2011.
- Bhasin B, Urekli HM, Atta MG: Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol 4: 235-244, 2015.
- Cianciolo RE, Mohr FC: Urinary System. In: Jubb, Kennedy & Palmer's Pathology of Domestic Animals: Volume 2, pp. 376-464.e371. 2016
- Forshaw D, Horwitz AM, Ellard K, Friend JA, Greed L, Metz M: Hyperoxaluria, hyperglycoluria and renal oxalosis in Gilbert's potoroos (Potorous gilbertii). Aust Vet J 95: 250-258, 2017.
- Jacob RH, Peet RL: Acute oxalate toxicity of sheep associated with slender iceplant (Mesembryanthemum nodiflorum). Australian Veterinary Journal 66: 91-92, 1989.
- McKenzie RA, Bell AM, Storie GJ, Keenan FJ, Cornack KM, Grant SG: Acute oxalate poisoning of sheep by buffel grass (Cenchrus ciliaris). Australian Veterinary Journal 65: 26, 1988.
- Means C: Insoluble Oxalates. In: Clinical Veterinary Toxicology, ed. Plumlee KH, pp. 340-341. Mosby, Inc. , Saint Louis, 2004.
- Pickrell JA, Oehme F: Soluble Oxalates. In: Clinical Veterinary Toxicology, ed. Plumlee KH, pp. 345-346. Mosby, Inc., Saint Louis, 2004.
- Rahman MM, Abdullah RB, Wan Khadijah WE: A review of oxalate poisoning in domestic animals: tolerance and performance aspects. J Anim Physiol Anim Nutr (Berl) 97: 605-614, 2013.
- Schepers MSJ, van Ballegooijen ES, Bangma CH, Verkoelen CF: Oxalate is toxic to renal tubular cells only at supraphysiologic concentrations. Kidney Int 68: 1660-1669, 2005.
- Speight KN, Boardman W, Breed WG, Taggart DA, Woolford L, Haynes JI: Pathological features of oxalate nephrosis in a population of koalas (Phascolarctos cinereus) in South Australia. Vet Pathol 50: 299-307, 2013.
- Letko A, Dijkman R, Strugnell B, Häfliger IM, Paris JM, Henderson K, Geraghty T, Orr H, Scholes S, Drögemüller C. Deleterious AGXT Missense Variant Associated with Type 1 Primary Hyperoxaluria (PH1) in Zwartbles Sheep. Genes (Basel). 2020 Sep 29;11(10):1147.
- Botha CJ, Truter M, Bredell T, Lange L, Mülders MS. Putative Aspergillus niger-induced oxalate nephrosis in sheep. J S Afr Vet Assoc. 2009 Mar;80(1):50-3.