CASE 2: 18-2108 (4117382-00)
Signalment:
Fourteen year-old, black Percheron gelding (Equus ferus caballus)
History:
A previously healthy gelding that received routine veterinary and dental care 6 days prior to death. The gelding was hitched to a cart at an event when it suddenly refused to go forward and within seconds, dropped to the ground. After a few spasms, it was declared dead.
Gross Pathology:
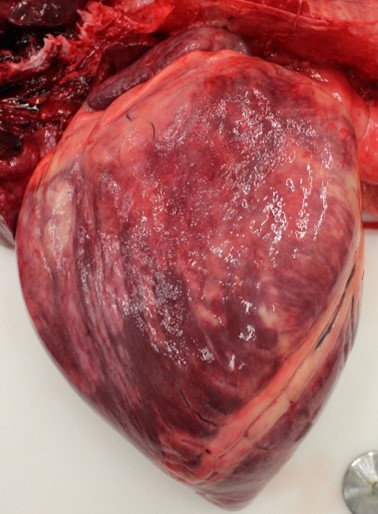
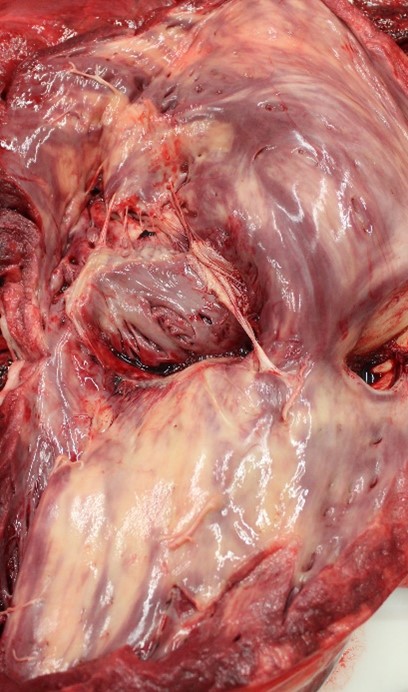
The horse was in good physical condition, well-fleshed, but not obese. The myocardium of the right atrium, right ventricle, and interventricular septum were streaked with numerous, well demarcated, white striae that extended transmurally from epicardium to endocardium. Adjacent papillary muscles were variably streaked as well. The left ventricular wall contained variably sized (1-5 cm diameter), irregularly round, well demarcated, bright white foci. Additionally, the epiglottis, larynx, and tracheal mucosa were speckled with petechial hemorrhages and the lungs were markedly congested.
Laboratory results:
None.
Microscopic description:
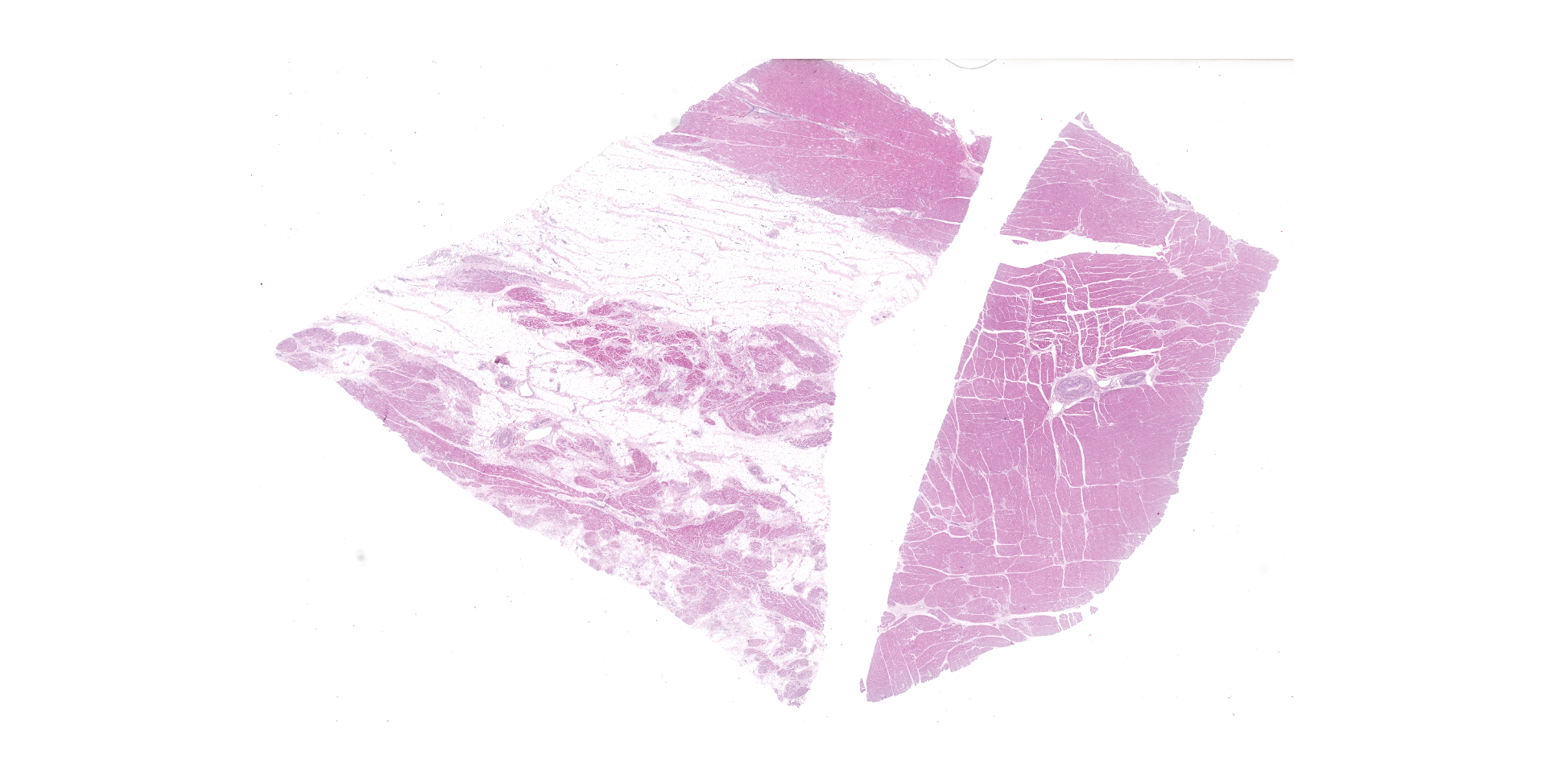
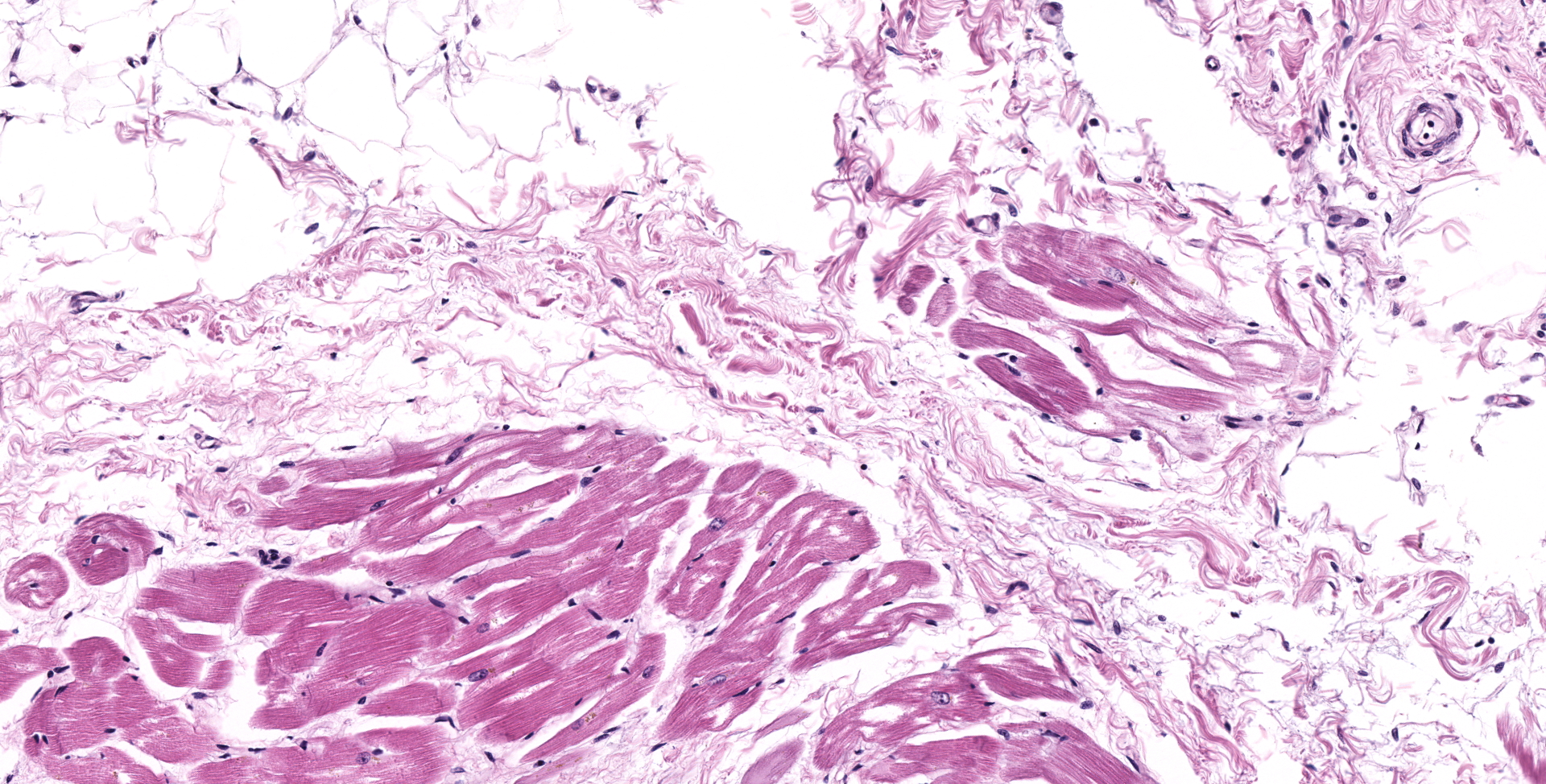
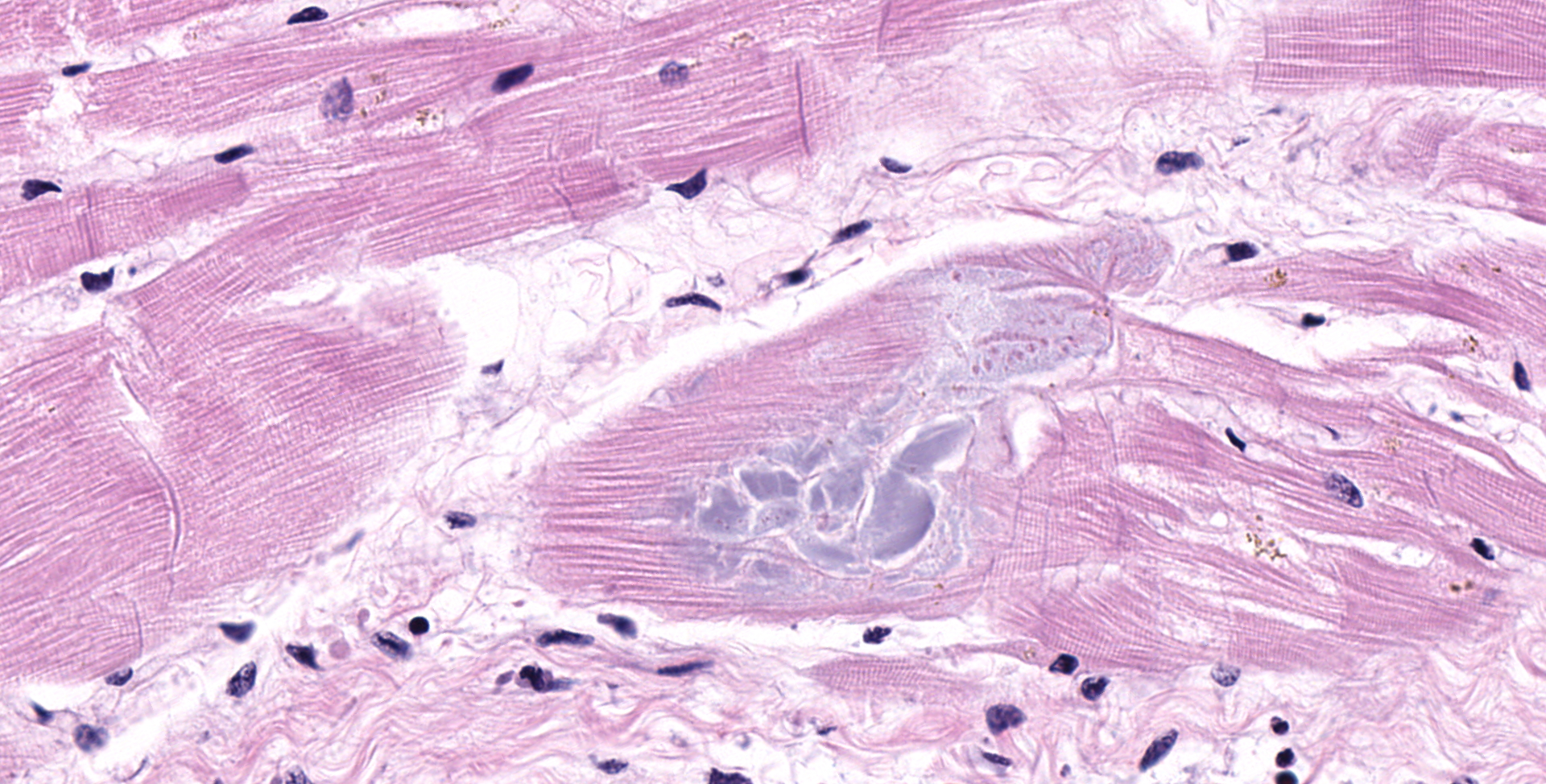
HEART (right ventricular wall and interventricular septum): Up to 75% of the right ventricular myocardium is replaced by adipose tissue, proliferating fibroblasts, and collagenous connective tissue. The tunica of most myocardial arteries is thickened up to 3-4 times normal by a circumferential proliferation of smooth muscle cells and collagen fibers (hypertrophy and hyperplasia), and most are surrounded by variable amounts of collagen fibers. Numerous myocardial fibers are swollen and pale, often fibrillar or fragmented, frequently contain 1-2 large vacuoles, and some exhibit significant variation of the diameter (atrophy). In another less affected section (interventricular septum), there is mild multifocal loss of myofibers and replacement by fibrous connective tissue. Randomly scattered throughout both sections, myofibers contain glassy, pale basophilic, PAS-positive, sarcoplasmic material that expands and sometimes completely replaces the fiber.
SKELETAL MUSCLE (tissue not submitted): Up to 60% of the myofibers display the following changes; diffuse pallor, loss of cross striations, sarcoplasmic swelling, and internalized nuclei. These fibers are infiltrated by few to moderate numbers of lymphocytes, macrophages, and plasma cells. There is accumulation of PAS-positive sarcolemmnal vacuoles in degenerative and normal myofibers. These vacuoles form coalescing aggregates that can completely replace the myofiber.
Contributor's morphologic diagnosis:
HEART (right ventricular free wall and interventricular septum):
1. moderate to marked arterial medial hyperplasia and hypertrophy;
2. mild to marked extensive myofiber degeneration and loss, with interstitial fibrosis and fatty infiltration;
3. mild to moderate myofiber accumulation of PAS-positive material
Contributor's comment:
Polysaccharide
storage myopathy (PSSM) in this Percheron gelding was confirmed by the
identification of PAS-positive intrasarcoplasmic polysaccharide inclusions in
multiple muscles and the heart.4,10 The distribution of changes in
the heart were consistent with chronic vascular disease with secondary cardiac
remodeling as opposed to a sequel of myocarditis.3 Presumably,
myocardial degeneration, fatty change, and interstitial fibrosis, can impact
the conduction system by blocking conducting pathways or forming ectopic foci
of electrical excitation, resulting in sudden death.3,8
PSSM
is a condition caused by an autosomal dominant, gain of function, missense
mutation (R309H) in the glycogen synthase gene (GYS1).7,8 GYS1, a
ubiquitously expressed enzyme, has a high expression in tissues with increased
glycogen demand and metabolism, such as skeletal and cardiac muscle.7
While the pathogenesis is unclear, mutation in this enzyme results in
accumulation of amylase-resistant alpha-crystalline polysaccharide.4,8,10,13
In humans, a similar mutation in GYS1, can present with a cardiac phenotype
with cardiac failure or dysrhythmias.11 In horses, PSSM has a wide
range of clinical presentations, from no clinical signs, to muscle pain,
shivers, ataxia, severe weakness, exertional rhabdomyolysis, and recumbency.8,10,13
Diagnostic criteria for PSSM is controversial, but common criteria include the
presence of PAS-positive +/- amylase resistant sarcoplasmic inclusions,
subsarcolemmal glycogen aggregates, central cytoplasmic glycogen aggregates, in
the presence of myopathic changes.4,13
Causes of myocardial disease in horses include viral infections (Equine Influenza, Equine Viral Arteritis, Equine Infectious Anemia, Equine Herpes Virus 1), bacterial infections (Streptococcus spp, Staphylococcus spp, Salmonella spp, Clostridium spp), parasitic infection (Strongylus vulgaris, Onchocerca spp), ingestion of cardiotoxic plants (Nerium oleander, Digitalis purpurea, Convallaria majalis, Rhododendron spp), ingestion of ionophore antibiotics (monensin, lasalocid, salinomycin), cantharidin toxicosis (blister beetle), and vitamin E/selenium deficiency.3,10 The etiology of chronic myositis and myocardial fibrosis in horses remains unknown, although any cause of myocarditis can result in myocardial scarring.3,10 This horse had no known history of disease or toxin exposure. Additionally, to our knowledge, there have been no confirmed reports of conduction defects in horses with PSSM and few reports of polysaccharide inclusions in the myocardium.8,15 Percherons, Belgians, and other Draft horses have a particularly high prevalence of both type I polysaccharide storage myopathy and involvement of cardiac muscle.8,13,15 In mice, glycogen storage myopathy and an over-expression of AMP-activated protein kinase results in conduction defects. The proposed mechanism of cardiac failure in humans and mice is distortion of the annulus fibrosis by vacuole-laden ventricular myocytes which allows an electrical impulse to by-pass the atrioventricular node and independently activate the ventricular myocardium.1
Contributing Institution:
University
of Connecticut
Connecticut Veterinary Medical Diagnostic Laboratory
Department of Pathobiology and Veterinary Science College of Agriculture, Health and Natural Resources
http://patho.uconn.edu
JPC diagnosis:
Heart, myocardium: Myofiber cytoplasmic glycogen-like inclusions, numerous, with multifocal to coalescing myofiber degeneration and loss, with marked fibrofatty infiltration.
JPC comment:
This case generated lively discussion about the fibrofatty replacement of myocytes, and whether this was likely a result of the EPSSM, or potentially a different process. Previous investigations in horses with PSSM have found sporadic fatty infiltration and replacement of myocytes, with a particularly severe case found in a homozygous horse.2 However, the clinical history of sudden collapse and death, the fibrofatty infiltration of the right ventricle, right atrium, and interventricular septum may also be consistent with arrhythmogenic right ventricular cardiomyopathy (ARVC). Reported cases have had patchy to diffuse fibrofatty infiltration and replacement of myocytes, few islands of adipose tissue within a loose collagenous stroma, with vacuolated and isolated or disrupted Purkinje fibers.5,9
Glycogen synthase (GS) is covalently regulated at nine phosphorylation sites which can be modified by various kinases. The kinases with the most significant contribution to decreased GS activity are glycogen synthase kinase 3b (GSK3b) and AMP-activated protein kinase (AMPK). Phosphorylation at four specific sites depress the enzyme activity than much more than the other five sites. Horses with homozygous mutant alleles had higher GS expression than wild types, and also had increased G6P-independent activity. As increased phosphorylation reduces GS activity, the elevation of GS activity remains despite increased phosphorylation of GS.6
Additional research examined the mitochondrial function of affected myocytes using high resolution respirometry (HRR), which has been used to detect mitochondrial dysfunction in humans. Certain mitochondrial dysfunctions have been associated with glycogen storage disease (GSD) in humans, such as GSD type II (Pompe disease), and GSD V (McArdle disease). While only a small group of affected horses was examined, there was a decrease in oxidative phosphorylation and electron transfer capacities in PSSM horses, with no substrate limitations evident. Different muscles examined (gluteus medius, triceps brachii) had different histopathologic appearances and levels of mitochondrial dysfunction, proposed to be due to different compositions and use of the muscle groups.12
PSSM that is due to GYS1 mutation has been categorized as PSSM type 1, and disproportionately affects Quarter horses, European-derived draught breeds, and related stock breeds. However, there remains a large population of cases of PSSM that are not correlated with GYS1 mutation, more often Warmblood and Arabian horses, and has been defined as PSSM type 2. A recently available genetic test for PSSM type 2 examines single nucleotide polymorphism (SNP) variants in the genes myotilin (MYOT), filamin C (FLNC) myozenin (MYOZ3). Unfortunately, in independent testing, there is no statistically significant correlation between gene status and diagnosis of PSSM type 2.14
References:
1. Arad M, Moskowitz IP, Patel VV, et al. Transgenic miceoverexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003; 107:2850?2856.
2. Barrey E, Mucher E, Jeansoule N, et al. Gene expression profiling in equine polysaccharide storage myopathy revealed inflammation, glycogenesis inhibition, hypoxia and mitochondrial dysfunctions. BMC Veterinary Research. 2009;5:29.
3. Buergelt, Claus D. Equine Cardiovascular Pathology: an Overview. Animal Health Research Reviews, 2003; 4 (2): 109?129.
4. Firshman, A. M, et al. Comparison of Histopathologic Criteria and Skeletal Muscle Fixation Techniques for the Diagnosis of Polysaccharide Storage Myopathy in Horses. Veterinary Pathology. 2006; 43 (3): 257?269.
5. Freel KM, Morrison LR, Thompson H, Else RW. Arrhythmogenic right ventricular cardiomyopathy as a cause of unexpected cardiac death in two horses. Veterinary Record. 2010; 166:718-722.
6. Maile CA, Hingst JR, Mahalingan KK, et al. A highly prevalent equine glycogen storage disease is explained by constitutive activation of a mutant glycogen synthase. Biochimica et Biophysica Acta. 2016;1861(1):3388-3398.
7. McCue ME, Valberg SJ, Miller MB, et al. Glycogen synthase (GYS1) mutation causes a novel skeletal muscle glycogenosis. Genomics. 2008; 91:458?466.
8. Naylor, et al. Evaluation of Cardiac Phenotype in Horses with Type 1 Polysaccharide Storage Myopathy. Journal of Veterinary Internal Medicine. 2012; 26(6): 1464?1469.
9. Raftery AG, Garcia NC, Thompson H, Sutton DGM. Arrhythmogenic right ventricular cardiomyopathy secondary to adipose infiltration as a cause of episodic collapse in a horse. Irish Veterinary Journal. 2015;68:24.
10. Robinson WF, Robinson NA. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol. 3. Philadelphia, PA: Elsevier, 2016:12?14.
11. Soliman OI, van der Beek NA, van Doorn PA, et al. Cardiac involvement in adults with Pompe disease. J Intern Med. 2008; 264:333?339.
12. Tosi I, Art T, Cassart D, et al. Altered mitochondrial oxidative phosphorylation capacity in horses suffering from polysaccharide storage myopathy. Journal of Bioenergetics and Biomembranes. 2018;50:379-390.
13. Valentine, B. A, and B. J Cooper. Incidence of Polysaccharide Storage Myopathy: Necropsy Study of 225 Horses. Veterinary Pathology. 2005; 42(6); 823?827.
14. Valberg SJ, Finno CJ, Henry ML, et al. Commercial genetic testing for type 2 polysaccharide storage myopathy and myofibrillar myopathy does not correspond to a histopathologic diagnosis. Equine Veterinary Journal. 2020. https://doi.org/10.1111/evj.13345
15. Valentine, B. A., et al.Severe Polysaccharide Storage Myopathy in Belgian and Percheron Draught Horses. Equine Veterinary Journal. 1997; 29(3); 220?225.