WSC 22-23
Conf 6
CASE III:
Signalment:
8-month old, male, Birman cat (Felis catus)
History:
Animal referred to the Neurology Unit of the Small Animal Hospital (School of Veterinary Medicine, University of Glasgow) with a 3 month history of hind limb weakness and hyporexia.
On clinical examination, the animal showed weakness in all limbs and urinary incontinence.
Clinical chemistry Hyperglobulinaemia, hyperproteinaemia.
Neurological examination revealed mild right sided head tilt, tremors of the head, CP severely delayed right side, tetra-ataxia and tetraparesis. Spinal and cranial nerve reflexes were normal.
MRI investigation identified ventriculomegaly, perivascular and white matter extensive oedema.
The owners elected euthanasia and authorised postmortem collection of the brain for histological evaluation.
Gross Pathology:
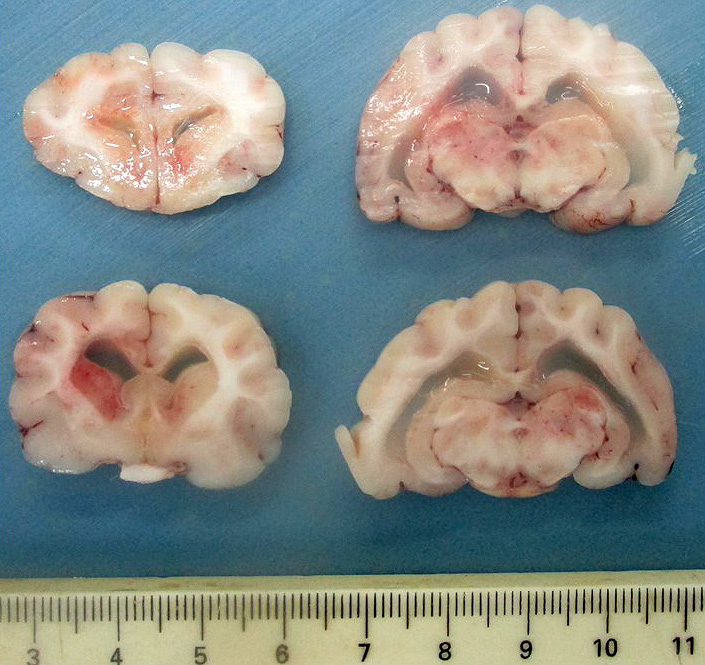
Upon dissection of the fixed brain, there is moderate bilateral dilation of the ventricles, with accumulation of moderate amount of pale translucent gelatinous fluid.
Laboratory Results:
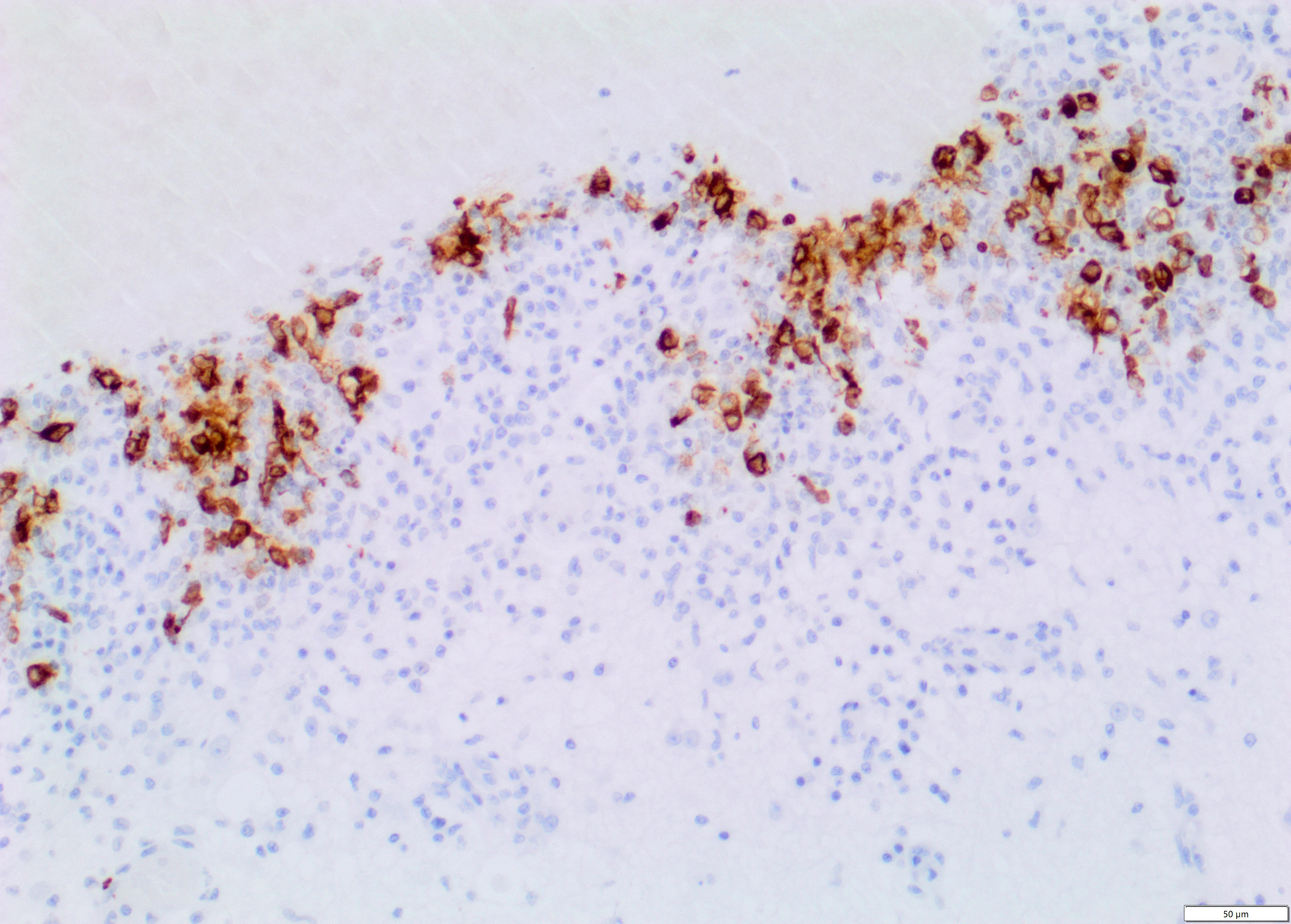
Immunohistochemistry for Feline Coronavirus (FCoV) confirmed the presence of FCoV antigen within the cytoplasm of macrophages in the inflammatory infiltrates.
Microscopic Description:
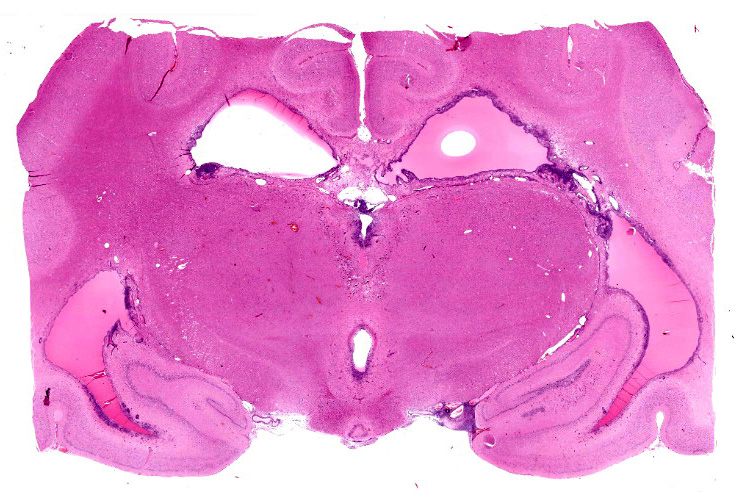
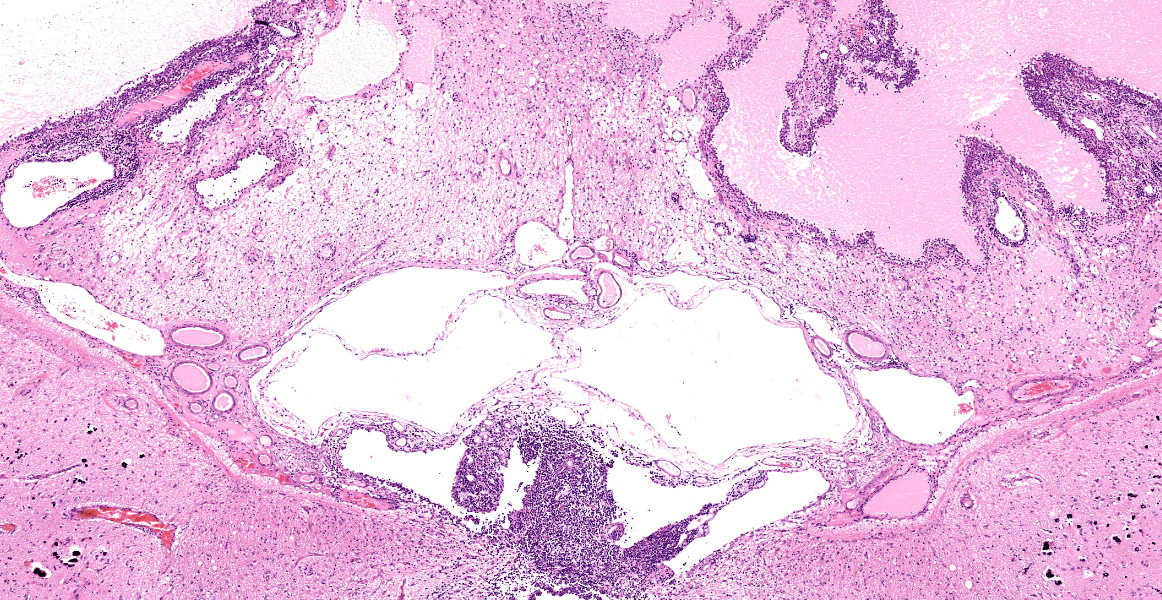
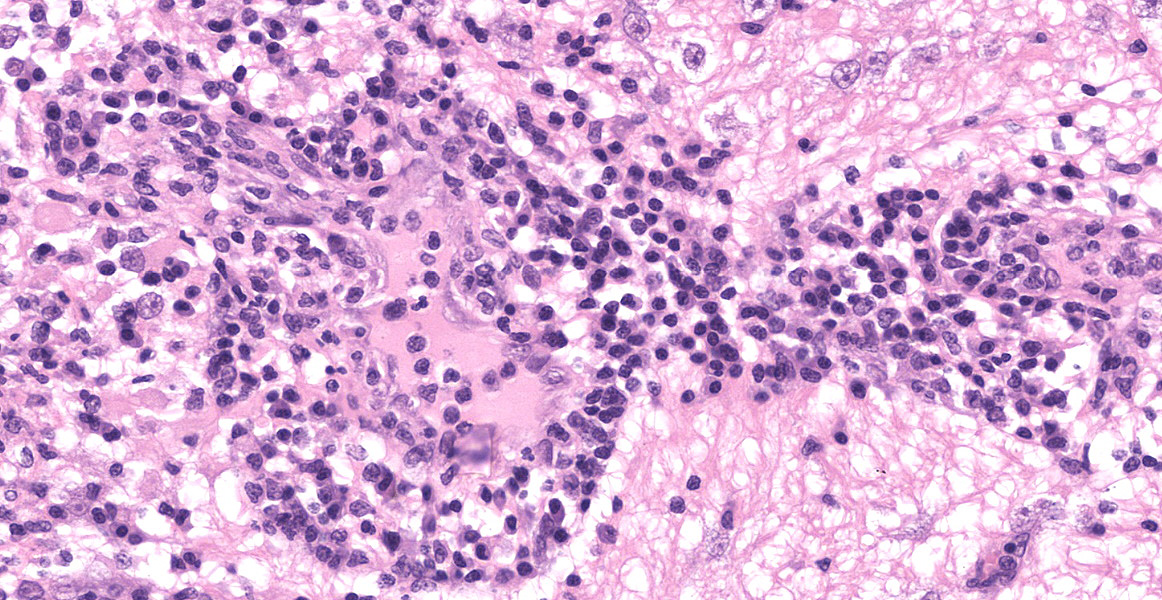
Section of brain at the level of the thalamus. Bilaterally affecting the lateral ventricles there are diffuse inflammatory changes effacing the ependymal lining and variably extending into the subependymal neuropil. Inflammation is dominated by macrophages admixed with scattered neutrophils, and variable numbers of plasma cells, and lymphocytes. Multifocal perivascular (cuffs up to 5 layers) and in some instances mural inflammatory infiltrates dominated by lymphocytes and plasma cells and variable numbers of macrophages affect small to medium sized vessels (venules) in the periventricular neuroparenchyma. Bilaterally the periventricular neuroparenchyma medial to the upper portion of the ventricles is partially effaced by accumulation of eosinophilic proteinaceous fluid with associated sparse infiltration of foamy macrophages admixed with lymphocytes and plasma cells. Multifocally, the periventricular grey and white matter is irregularly vacuolated (spongy change) with few scattered spheroids.
Bilaterally, the lateral ventricles are distended by moderate to large amounts of eosinophilic proteinaceous fluid.
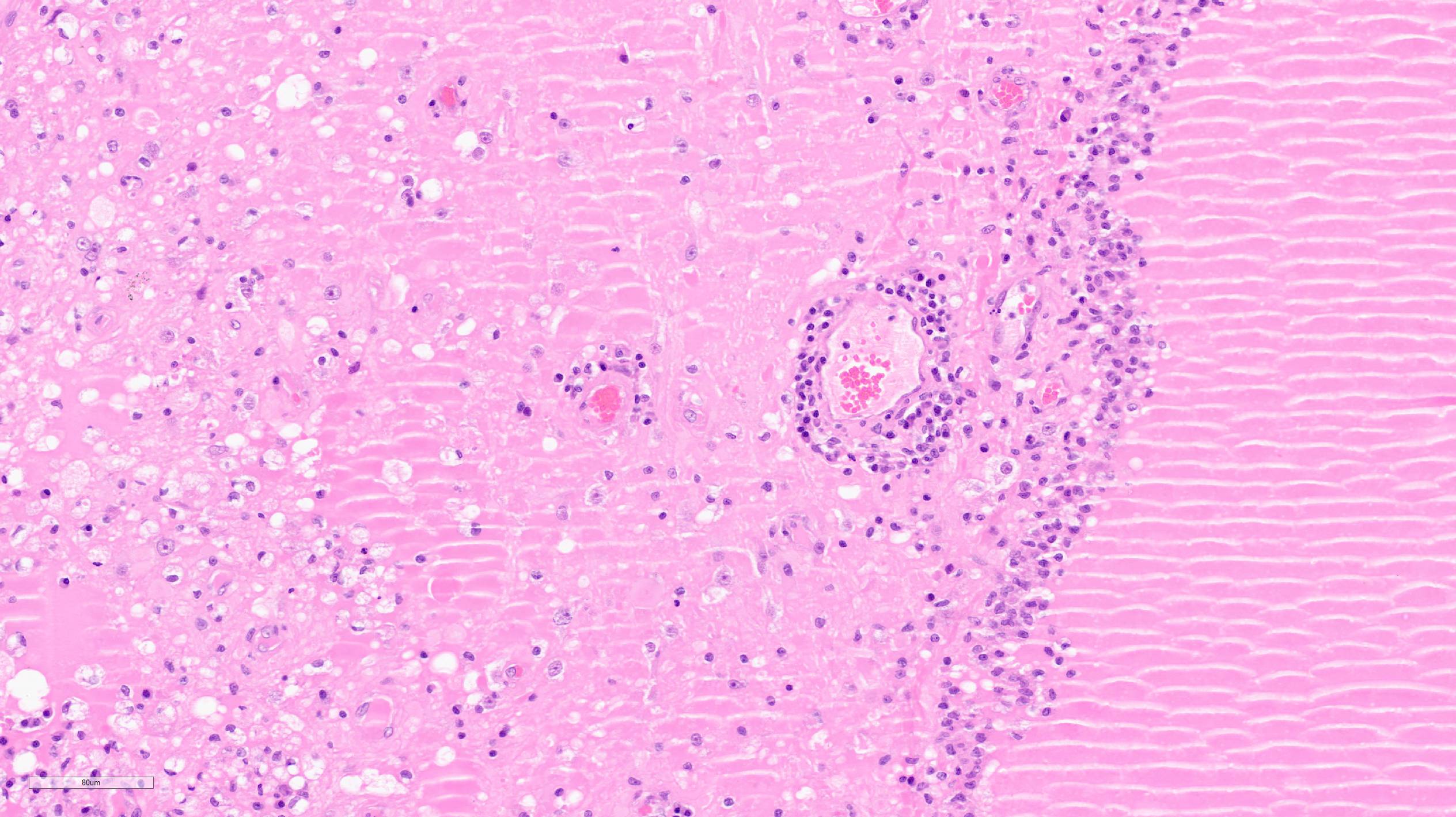
Multifocally, within the hippocampus, in both the pyramidal and molecular layers, there are numerous deposits of coarse globular to laminar basophilic material (mineralisation) variably obliterating the wall and lumen of small capillary vessels and in some instances apparently associated with neurones.
In the leptomeninges on the ventral aspect of the thalamus there is variable mild to moderate infiltration of lymphocytes, macrophages, neutrophils plasma cells with sparse and perivascular distribution and moderate necrosis.
Immunohistochemistry for Feline Coronavirus (FCoV) confirmed the presence of FCoV antigen within the cytoplasm of macrophages in the inflammatory infiltrates.
Note: Due to availability of material in the original samples, the slides have been prepared from two different tissue blocks. Inflammatory changes in the leptomeninges ventral to the thalamus are mild in one section and more prominent in the other section. In the latter section, mineralisation is also variably present in the periventricular neuroparenchyma in the thalamus.
Contributor’s Morphologic Diagnoses:
Brain: periventricular encephalitis, granulomatous/pyogranulomatous and lymphoplasmacytic, chronic extensive marked.
Meningitis, pyogranulomatous necrotising and lymphoplasmacytic, chronic multifocal moderate.
Hippocampus, capillary vessels and neurones, mineralisation, multifocal moderate.
Contributor’s Comment:
Feline Coronaviruses (FCoVs) are pleomorphic, enveloped, single-stranded positive sense RNA viruses that belong to the family Coronaviridae, order Nidovirales, and genus Alphacoronavirus, species Alphacoronavirus 1.13 FCoVs occur as 2 biological pathotypes: feline enteric coronavirus (FECV), defined as the ‘‘ubiquitous enteric biotype,’’ and feline infectious peritonitis virus (FIPV), the ‘‘virulent biotype” that causes FIP in individual cats.13, 25 FCoVs can also be divided into two antigenically distinct serotypes (I and II) based on cell culture cytopathic effect and other features, and type I FCoV are more frequently associated with FIP than type II FCoV.25 Given their close genetic relationship, the viral strains are serologically indistinguishable and difficult to differentiate by routine laboratory testing, making an accurate clinical diagnosis of FIP often difficult.24
FCoVs are ubiquitous in cats, but the disease FIP is sporadic, and purebred, young, intact male cats appear to be more susceptible.25 It is transmitted via the faecal-oral route and primarily infect enterocytes.13 Whereas FECVs replicate mainly in intestinal epithelium and are shed in faeces, FIPVs replicate efficiently in monocytes and induce systemic disease. The host’s genetics and immune system also play important roles.13, 25
|
Table 3-1: coronaviruses affecting different species and their associated disease/lesions |
|||
|
Animal species |
Virus |
Associated disease/lesions |
References |
|
Feline |
Feline infectious peritonitis virus (FIPV) |
Multisystemic granulomatous/pyogranulomatous disease with vasculitis, serositis, meningoencephalitis, uveitis/ophthalmitis |
12,14,22,23 |
|
Feline enteric coronavirus (FECV) |
Enteritis with diarrhoea in kittens |
19 |
|
|
Canine |
Canine Coronavirus (CCV) |
Nonfatal enteritis in puppies |
2,4 |
|
Ferret |
Ferret enteric coronavirus (FRECV) |
Epizootic catarrhal enteritis |
24 |
|
Ferret systemic coronavirus (FRSCV) |
Systemic pyogranulomatous inflammation similar to FIP in cats |
5,7 |
|
|
Bovine |
Bovine coronavirus (BCV) |
Severe diarrhoea and respiratory disease in calves and diarrhoea (winter dysentery) in adults |
1,15 |
|
Mouse |
Mouse hepatitis virus (MHV) |
Enteritis, hepatitis and demyelinating encephalomyelitis |
3 |
|
Porcine |
Porcine endemic diarrhoea virus (PEDV) |
Atrophic enteritis in neonatal piglets |
10 |
|
Porcine hemagglutinating encephalomyelitis virus (PHEV) |
Lymphoplasmacytic perivascular cuffing in the brain, and stomach muscularis and submucosa |
13 |
|
|
Transmissible gastroenteritis virus (TGEV) |
Enteritis with diarrhoea |
11 |
|
|
Rat |
Rat coronavirus (RCV) |
Rhinitis, tracheitis, pneumonitis in young rats |
17,21 |
|
Rat sialodacryoadenitis virus (SDAV) |
Sialoadenitis, dacryoadenitis, rhinitis, tracheitis, bronchitis/bronchiolitis and alveolitis |
21,25 |
|
|
Chickens |
Avian infectious bronchitis virus (IBV) |
Tracheobronchitis, nephritis |
9 |
|
Turkeys |
Turkey coronavirus (TCV)/Bluecomb virus |
Enteritis, cyanosis, severe growth depression |
8 |
|
Humans |
Middle East respiratory syndrome coronavirus (MERS-CoV) |
Mild respiratory illness to severe pneumonia and multi-organ failure |
16 |
|
Severe acute respiratory syndrome coronavirus (SARS-CoV) |
Severe acute respiratory syndrome |
20 |
|
|
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
Severe acute respiratory syndrome / COVID-19 |
26,27 |
|
FIP is characterized by fibrinous serositis, with protein-rich effusions in the body cavities of affected cats (effusive or “wet” FIP), as well as granulomatous-necrotising lesions, periphlebitis, and granulomatous and pyogranulomatous inflammatory lesions in several organs, especially, liver, kidney, spleen, leptomeninges, and eyes (non-effusive or “dry” FIP)16 However, mixed forms are probably common.25
Lesions in the CNS are typically oriented toward the surface and target the leptomeninges, ependyma, choroid plexus, and neuroparenchyma.24 The presence of eosinophilic proteinaceous material within the lateral ventricles observed in this case is interpreted as hydrocephalus, which is a common secondary gross finding described in cases of FIP.20, 24, 25 Inflammation within or around the ventricular system may lead to obstruction of cerebrospinal fluid and cause secondary hydrocephalus.24
It can be speculated that the presence of multifocal mineral deposits within the hippocampus is a consequence of hypercalcaemia in this cat, which has been reported in association with a few infectious diseases featuring granulomatous inflammation (including FIP). Macrophages can synthesize calcitriol from calcidiol without any negative feedback regulation, thus leading to hypercalcaemia and subsequently metastatic mineralisation.6
Contributing Institution:
Division of Pathology, Public Health and Disease Investigation
Veterinary Diagnostic Services
School of Veterinary Medicine
College of Medical, Veterinary and Life Sciences
University of Glasgow (Garscube Campus)
464 Bearsden Road
Glasgow G61 1QH, Scotland
https://www.gla.ac.uk/schools/vet/cad/
JPC Diagnosis:
Diencephalon: Ventriculitis, pyogranulomatous and lymphoplasmacytic, diffuse, severe, with hydrocephalus, phlebitis, choroid plexitis, periventricular necrosis and edema, meningitis, and mineralization.
JPC Comment:
Neurologic manifestations of feline infectious peritonitis have three general distribution patterns as described by Rissi: diffuse leptomeningitis; rhombencephalitis (or inflammation of the brainstem/cerebellum); and periventricular encephalitis, which is demonstrated in this case and was the most prevalent form in Rissi’s study of 26 cats affected by FIP in the central nervous system.
Feline coronavirus (FCoV) is extremely common in domestic cats, and it’s estimated that 25% of cats in single cat households and over 75% of cats within multi-cat households are infected.9 Of those infected, anywhere from 1 to 12% may develop FIP.9,14 As an RNA-virus, FCoV is prone to replication errors, and a mutation within the spike protein likely accounts for the ability of FIP to infect monocytes and macrophages and cause systemic infection.14 The spike protein has two subunits, S1 (receptor binding) and S2 (fusion), and a proteolytic cleavage site which, when cleaved, results in activation of the spike protein.14 In a study of 11 cats with FIP, unique mutations were consistently found in the S1/S2 cleavage site, which the authors speculate may make the protein more susceptible to cleavage by other enzymes.14
The internal mutation theory is the most widely accepted model of FIP pathogenesis and asserts that, in each patient, FCoV spontaneously mutates into the pathogenic non-contagious FIP.9 A variation of the internal mutation theory called the circulating virulent and avirulent theory asserts that certain strains are more likely to induce FIP, which may explain periodic clusters of FIP cases.9,14 Such a case series was documented in four cats from a single household who succumbed to FIP after being displaced due to a house fire and surrendered to a shelter. 9 The three cats which had viral genetic sequencing each had different mutation profiles within the S1/S2 gene. 9 The authors concluded that these cats likely had a unique FCoV strain prone to FIP-mutation, and the mutations in each cat may have been precipitated by significant physiologic stress. 9
Finally, rare cases of horizontal transmission have also been reported. At one Taiwanese shelter, 13 cats (28% of the population) succumbed to FIP over a one year time period.26 All cats were infected with type II FCoV, and the viruses all had identical S gene mutations.26 Those infected later in the year had additional unique mutations in the 3c gene indicating ongoing mutation.26 Additionally, the type II FCoV associated with FIP was isolated from both the feces and oronasal and conjunctival samples from infected cats, indicating horizontal transmission was likely responsible for the spread of the virus within the shelter.26
References:
- Bok M, Alassia M, Frank F, Vega CG, Wigdorovitz A, Parreño V. Passive immunity to control Bovine coronavirus diarrhea in a dairy herd in Argentina. Rev Arg Microb. 2018;50:23-30.
- Buonavoglia C, Decaro N, Martella V, et al. Canine Coronavirus Highly Pathogenic for Dogs. Emerg Infect Dis. 2006;12:492-494.
- Compton SR, Ball-Goodrich LJ, Johnson LK, Johnson EA, Paturzo FX, Macy JD. Pathogenesis of Enterotropic Mouse Hepatitis Virus in Immunocompetent and Immunodeficient Mice. Comp Med. 2004;54:681-689.
- Decaro N, Buonavoglia C. An update on canine coronaviruses: Viral evolution and pathobiology. Vet Microb. 2008;132:221-234.
- Doria-Torra G, Vidaña B, Ramis A, Amarilla SP, Martínez J. Coronavirus Infection in Ferrets: Antigen Distribution and Inflammatory Response. Vet Pathol. 2016;53:1180-1186.
- Finch NC. Hypercalcaemia in cats: The complexities of calcium regulation and associated clinical challenges. J Feline Med Surg. 2016;18:387-399.
- Garner MM, Ramsell K, Morera N, et al. Clinicopathologic Features of a Systemic Coronavirus-Associated Disease Resembling Feline Infectious Peritonitis in the Domestic Ferret (Mustela putorius). Vet Pathol. 2008;45:236-246.
- Guy JS. Turkey coronavirus is more closely related to avian infectious bronchitis virus than to mammalian coronaviruses: A review. Avian Pathol. 2000;29:207-212.
- Healey EA, Andre NM, Miller AD, Whitaker GR, Berliner EA. Outbreak of feline infectious peritonitis (FIP) in shelter-housed cats: molecular analysis of the feline coronavirus S1/S2 cleavage site consistent with a ‘circulating virulent-avirulent theory’ of FIP pathogenesis. JFMS Open Rep. 8(1):1-8.
- Ignjatovic J, Sapats S. Avian infectious bronchitis virus. Rev Sci Tech. 2000;19:493-508.
- Jung K, Saif LJ. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet J. 2015;204:134-143.
- Kim SH, Kim IJ, Pyo HM, Tark D-S, Song JY, Hyun BH. Multiplex real-time RT-PCR for the simultaneous detection and quantification of transmissible gastroenteritis virus and porcine epidemic diarrhea virus. J Virol Methods. 2007;146:172-177.
- Kipar A, Meli ML. Feline Infectious Peritonitis: Still an Enigma? Vet Pathol. 2014;51:505-526.
- Licitra BN, Millet JK, Regan AD, et al. Mutation in Spike Protein Cleavage Site and Pathogenesis of Feline Coronavirus. Em Infect Dis. 2013;19(7):1066-1073.
- Mora-Díaz JC, Piñeyro PE, Houston E, Zimmerman J, Giménez-Lirola LG. Porcine Hemagglutinating Encephalomyelitis Virus: A Review. Front Vet Sci. 2019;6:53.
- Myrrha LW, Silva FMF, Peternelli EF de O, Junior AS, Resende M, Almeida MR de. The Paradox of Feline Coronavirus Pathogenesis: A Review. Adv Virol. 2011;2011:1-8.
- Oma VS, Tråvén M, Alenius S, Myrmel M, Stokstad M. Bovine coronavirus in naturally and experimentally exposed calves; viral shedding and the potential for transmission. Virol J. 2016;13:100.
- Omrani AS, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog Glob Health. 2015;109:354-362.
- Parker JC, Cross SS, Rowe WP. Rat coronavirus (RCV): A prevalent, naturally occurring pneumotropic virus of rats. Archiv Gesamte Virusforschung. 1970;31:293-302.
- Pedersen NC, Allen CE, Lyons LA. Pathogenesis of feline enteric coronavirus infection. J Feline Med Surg. 2008;10:529-541.
- Pedersen NC. A review of feline infectious peritonitis virus infection: 1963-2008. J Feline Med Surg. 2009;11:225-258.
- Peiris JSM, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10:S88-S97.
- Percy DH, Barthold DW. Chapter 2: Rat. In: Pathology of Laboratory Rodents and Rabbits. Wiley Blackwell; 2016:125-127.
- Rissi DR. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J Vet Diagn Invest. 2018;30:392-399.
- Uzal FA, Blattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy & Palmer´s Pathology of Domestic Animals. 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:253-255.
- Wang YT, Su BL, Hseih LE, Chueh LL. An outbreak of feline infectious peritonitis in a Taiwanese shelter: epidemiologic and molecular evidence for horizontal transmission of a novel type II feline coronavirus. Vet Res. 2013; 44(57):1-9.
- Wise AG, Kiupel M, Maes RK. Molecular characterization of a novel coronavirus associated with epizootic catarrhal enteritis (ECE) in ferrets. Virology. 2006;349:164-174.
- Yoo D, Pei Y, Christie N, Cooper M. Primary Structure of the Sialodacryoadenitis Virus Genome: Sequence of the Structural-Protein Region and Its Application for Differential Diagnosis. Clin Diagn Lab Immunol. 2000;7:568-573.
- Zhang Y-Z, Holmes EC. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell. 2020;181:223-227.
- Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. 2020;579:270-273.