Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 19
25 March, 2020
CASE IV: LE-2 (JPC 4049442).
Signalment: Porcine, breed unspecified (Sus scrofa domestica), 14-weeks-old, neutered male, body weight 56.6 kg
History: This pig was submitted from a feeder-to-finish pig farm. Due to alterations of the skin a contagious disease was suspected. Therefore, the pig was euthanized and submitted for a complete post mortem examination. A more detailed clinical history was not available.
Gross Pathology: The carcass showed multiple cutaneous red macules and patches measuring up to 2 cm in diameter that were predominately located on the head, over the scapulae, as well as at the distal extremities and the anus.
Peripheral and visceral lymph nodes were enlarged measuring up to three times of their normal size. Their cut surfaces contained multiple slightly elevated white foci measuring 0.2 cm in diameter (indicative of a marked follicular hyperplasia) and displayed a red marginal coloration (consistent with a moderate blood resorption). Furthermore, both kidneys had multiple red foci measuring 0.1 cm in diameter that were restricted to the cortex; renal alterations were interpreted as a diffuse moderate acute glomerulonephritis. In addition, lungs showed a mild to moderate suppurative bronchopneumonia in both cranial lobes.
Laboratory results: PCR was positive for the detection of porcine Circovirus type 2 in tissue samples of brain, lungs and kidneys.
PCR was negative for the detection of classical swine fever virus in tissue samples f brain, lungs, lymph nodes, tonsils and spleen.
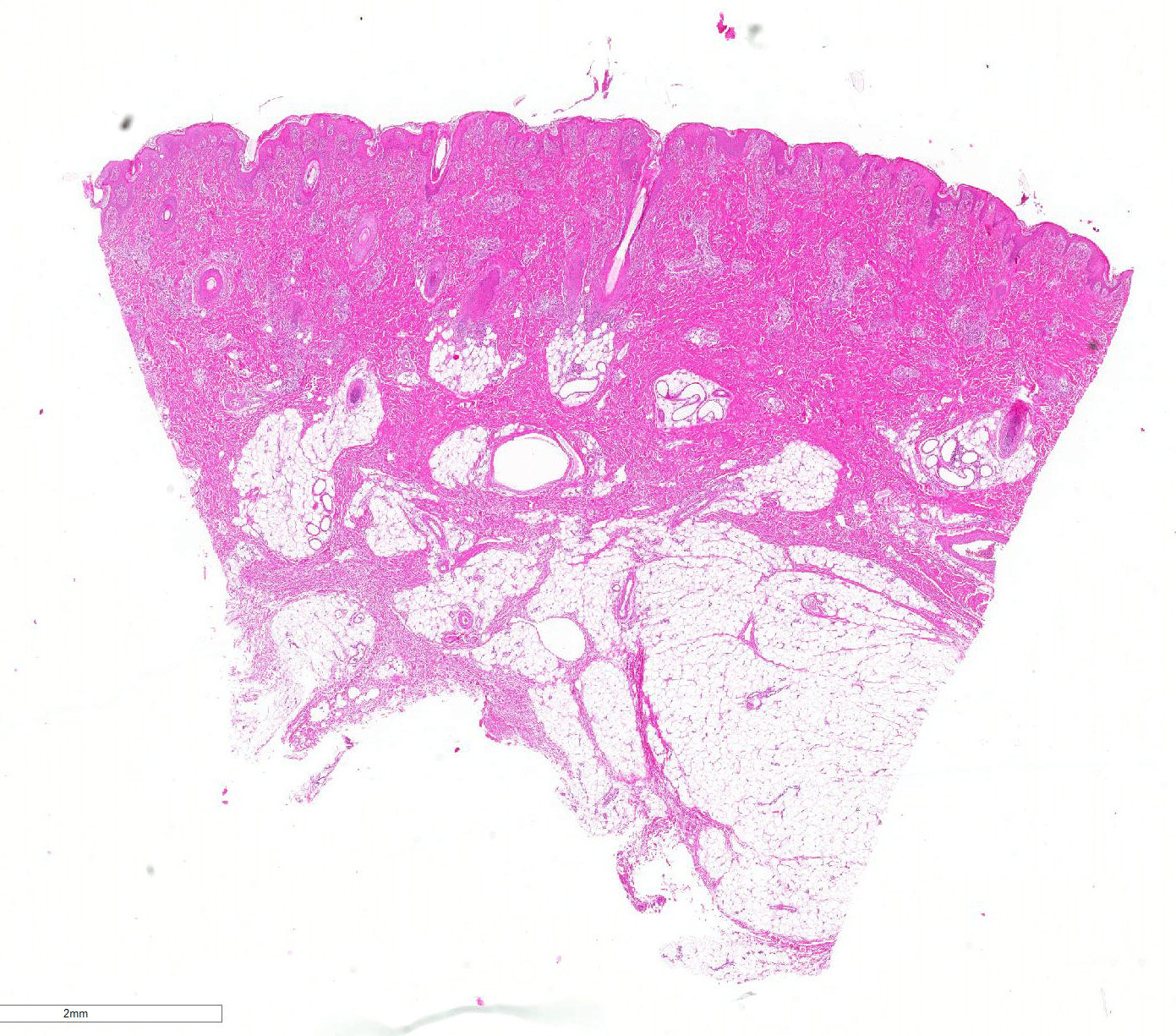
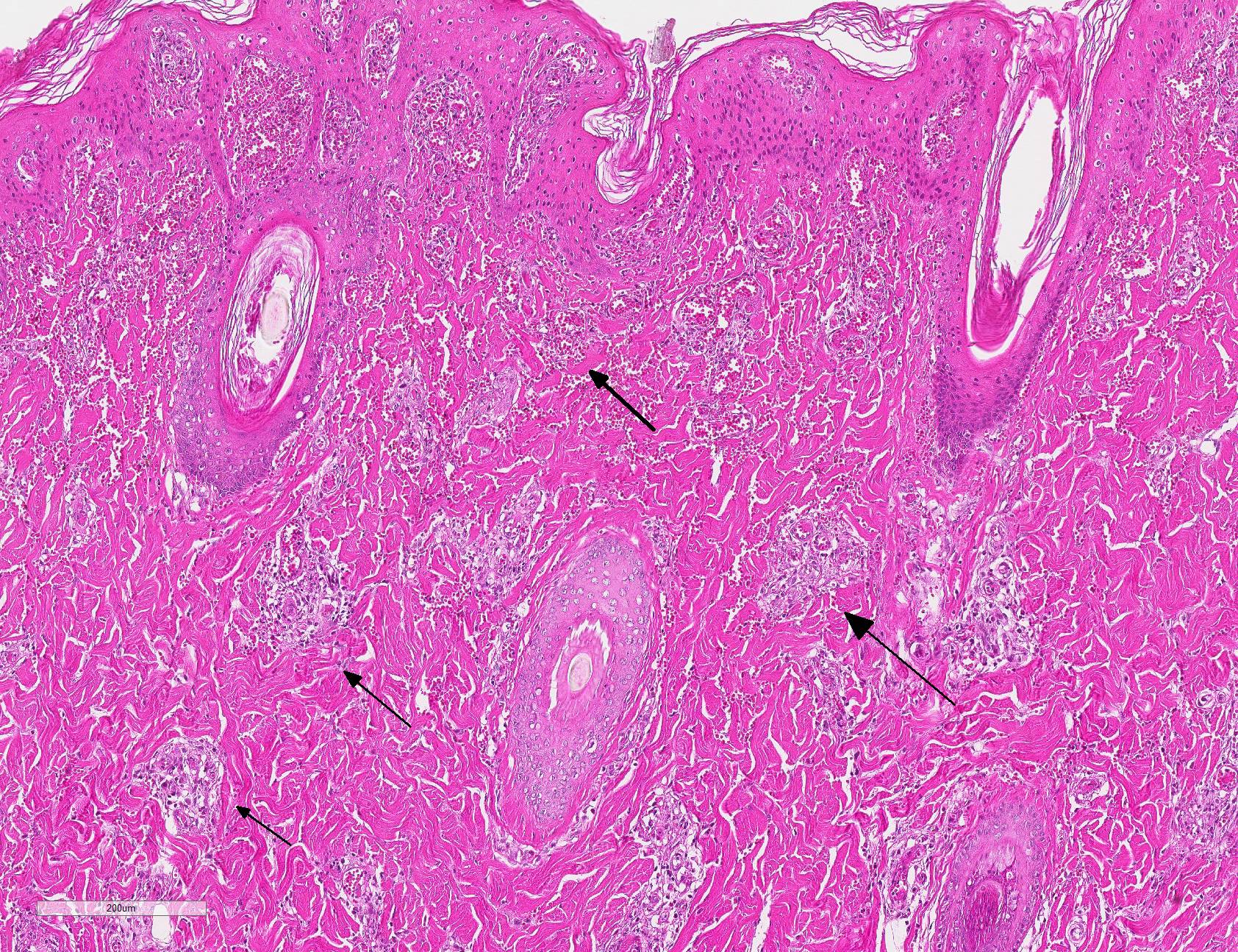
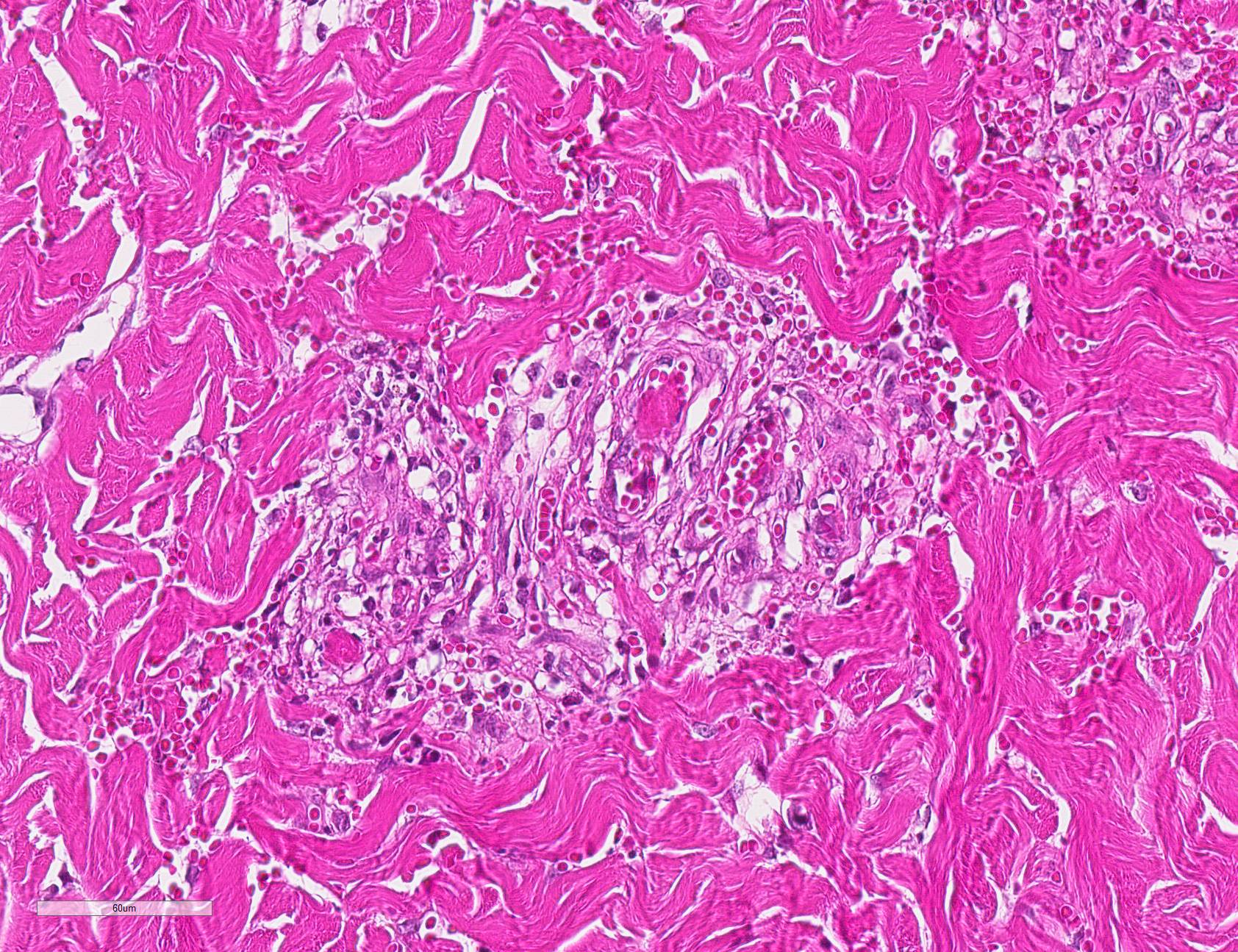
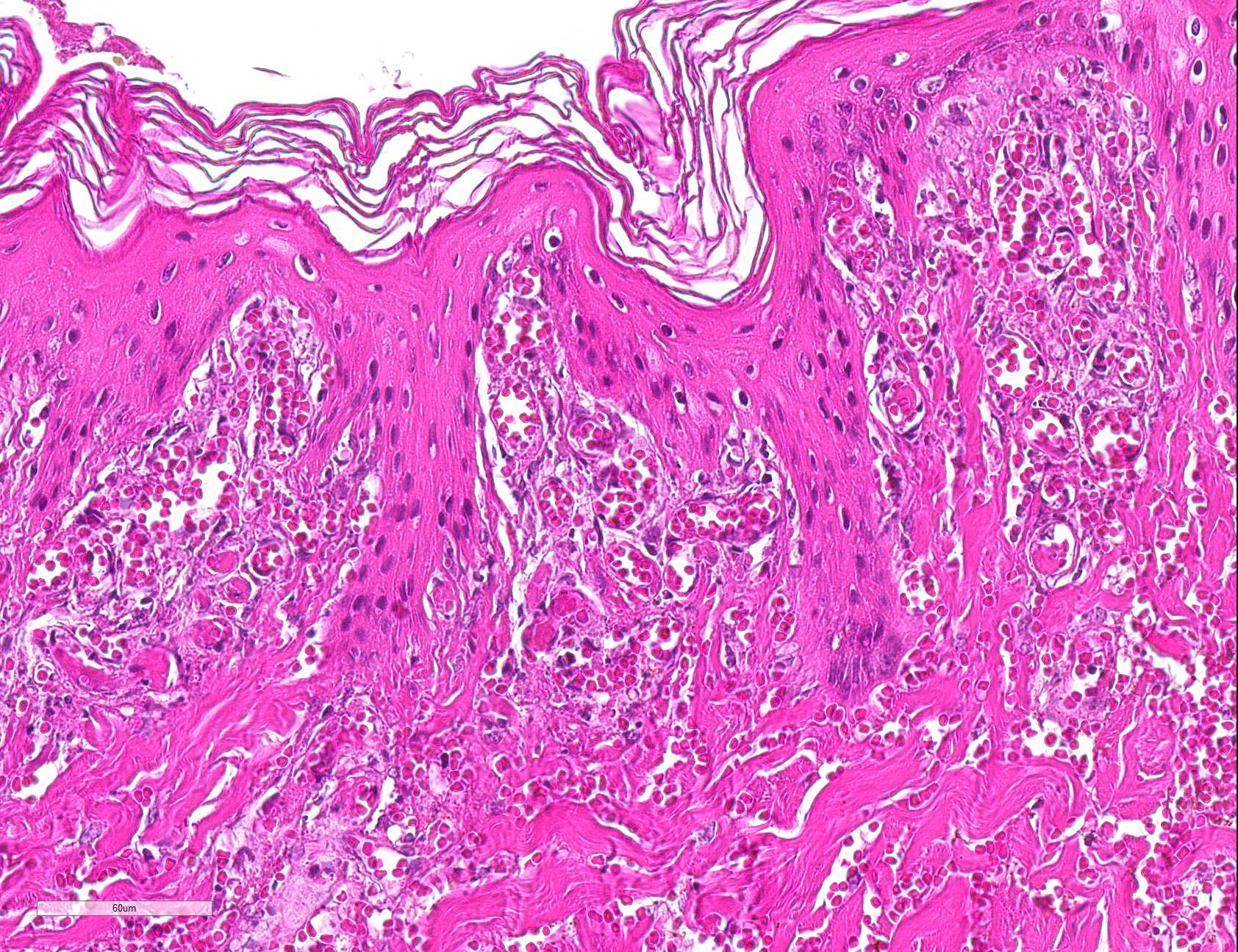
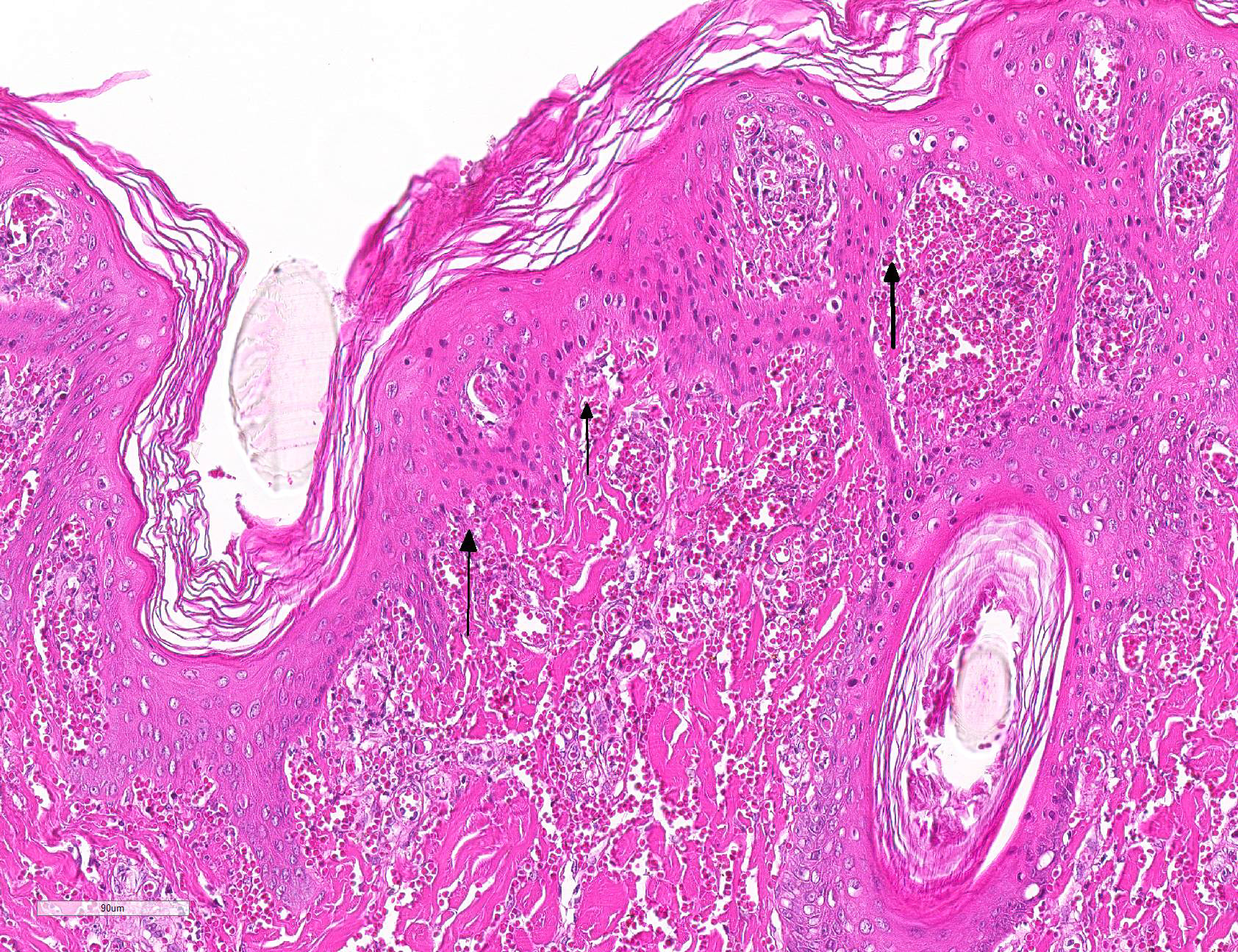
Microscopic Description: Haired skin: Multifocally, vascular walls of dermal capillaries, arterioles and venules showed deposition of homogenous eosinophilic material associated with loss of normal architecture interpreted as fibrinoid degeneration. Endothelial cells were swollen and bulged into vascular lumens (activated endothelial cells) or showed different stages of degeneration or necrosis (karyopyknosis and karyorrhexis). Some affected vessels were surrounded by a few extravasated erythrocytes (Fig. 1, arrowhead), and/or were cuffed by moderate numbers of inflammatory cells that extended within the adjacent dermis (Fig.1, asterisk). Some affected vessels contained intraluminal fibrin thrombi and/or moderate numbers of neutrophils. The inflammatory infiltrates consisted of predominately mononuclear cells (macrophages, lymphocytes, plasma cells) and a few neutrophils. Furthermore, a diffuse mild to moderate perivascular edema existed. The epidermis showed mild irregular epidermal hyperplasia and mild orthokeratotic basket-woven hyperkeratosis. In some slides, the described vascular lesions were also observed within the subcutis (Fig. 2, overview).
Contributor?s Morphologic Diagnosis:
Haired skin, dermis and subcutis: Perivasculitis and vasculitis, necrotizing and mixed-cellular, acute to subacute, multifocal, moderate, with perivascular hemorrhage and edema
Contributor?s Comment: Porcine circovirus type 2 (PCV2) belongs to the family Circoviridae, that encompass the two genera Circovirus and Gyrovirus. Porcine circovirus type 1 (PCV1) and type 2, beak and feather disease virus, canary, goose, pigeon, duck, finch and gull circovirus belong to the genus Circovirus. The genus Gyrovirus contains only the chicken anemia virus (CAV).13
The replication of the virions takes place within the nucleus of the host cell during the S phase of the mitosis by using the host cell DNA polymerase. PCV2 is reported as the smallest known pathogenic virus of our domestic animals with a size of approximately 16 to 20 nm in diameter.13
The porcine circovirus was first mentioned in 1974 from Tischer and colleagues.11 It had been isolated from a pig kidney cell line PK 15 as a contaminant. At this time it was thought to be a picornavirus.11 In the 1980s the same investigator group classified the virus as a porcine circovirus.12 It has an isometric shape. The DNA is a covalently closed circular molecule. Furthermore, DNA is single stranded and has a genome size of 1.76 kb.12
In 1997, Clark described a new pig disease that occurred in Canada and called it the Post-Weaning Multisystemic Wasting Syndrome (PMWS).3 Typical pathomorphologic findings are interstitial pneumonia characterized by mononuclear cellular infiltrates including predominately large macrophages and numerous multinucleated syncytial cells as well as enlarged lymph nodes characterized by a lymphoid depletion, an infiltration with large histiocytic cells and multinucleated syncytial cells.3 In some cases, intralesional histiocytic cells display intracytoplasmic basophilic inclusions (botryoid inclusion bodies).3,6 At this time the porcine circovirus has been suggested as the infectious agent.3
A comparison of the viruses of the pig kidney cell line PK 15 with those isolated from the animals with PMWS showed that they were antigenically distinct. Allan and colleagues designated the original PCV isolate from the pig kidney cell line PK 15 as PCV1 and the disease associated circovirus as PCV2.1
Retrospective studies showed the presence of PCV2 back to the 1970s in the United Kingdom pig population and at least since 1962 in Northern Germany.4,6 Nowadays, a global distribution within the pig population is known.7 PCV2 can be found in healthy as well as in diseased pigs.2 It is speculated that a breakout of a PCV2-associated disease is multifactorial in causality like host susceptibility, immune modulation, coinfection.7 Viruses spread via fecal-oral route, but vertical transmission has also been demonstrated.13
Opriessnig and Langohr summarized the diversity of PCV2-associated diseases in pigs.9 Those can be divided into subclinical PCV2 infection, PCV2-associated systemic infection, PCV2-associated respiratory disease, PCV2-associated enteritis, PCV2-associated reproductive failure and PCV2-associated porcine dermatitis and nephropathy syndrome (PDNS).8
In the present case, microscopic examination of enlarged lymph nodes revealed lymphocytic depletion as well as infiltration by numerous multinucleated giant cells (syncytial cells). Furthermore, lungs showed a marked diffuse interstitial non‑suppurative pneumonia and a moderate multifocal acute suppurative bronchopneumonia. The kidneys displayed histologically a multifocal moderate non-suppurative interstitial nephritis, a suppurative glomerulonephritis and a multifocal marked necrotizing vasculitis and perivasculitis.
PCV2-associated PDNS is characterized by typical gross lesions of the skin and the kidneys together with the hallmark microscopic lesions (systemic vasculitis and glomerulonephritis) and detection of PCV2 mRNA or antigen. Higgins mentioned PDNS also in association with drugs, other infectious agents and neoplasia.5
Therefore in the present case, PCV2-associated PDNS is most likely due to the gross lesions, histopathological findings and the detection of PCV2 mRNA within multiple tissues of this pig.
The proposed pathogenesis of PDNS is a type III hypersensitivity reaction with deposition of immune complexes in vessel walls and glomerular tuffs. In regard to the PCV2-associated PDNS, Rosell and collegues couldn?t always detect porcine circovirus nucleic acid in vascular and glomerular lesions.10 They supposed a transient deposition and a promptly removal of immune complexes or that the immune complexes lack of viral genome and only contain viral protein.
One of the major differential diagnoses of a disseminated acute vasculitis and glomerulonephritis is classical swine fever, in addition other viremic or septicemic diseases have to be ruled out. Further virological examination is required in such cases, i.e. PCR and/or virus culture, to definitively rule out the presence of a highly contagious and notifiable disease. The present case was negative for classical swine fever virus.
In case several animals of this farm may present with similar lesions, management practices, disinfection, control of coinfections and PCV2 vaccines should be recommended.8
Contributing Institution:
Institute of Pathology
Faculty of Veterinary Medicine
University of Leipzig
Director Professor H.-A. Schoon
An den Tierkliniken 33
04103 Leipzig
Germany
http://pathologie.vetmed.uni-leipzig.de/
JPC Diagnosis: Haired skin: Vasculitis, necrotizing, diffuse, severe, with thrombosis, hemorrhage, and multifocal epidermal necrosis.
JPC Comment: The contributor has provided an excellent review of the history of porcine circovirus-2 investigation. PCV-2 has made a number of appearances in the WSC demonstrating the wide spectrum of disease associated with this virus. Previous WSC submissions falling into this category include a two previous cases of PCV-associated myocarditis (WSC 2019, Conference 17 Case 2 and WSC 2016, Conf 8, Case 2), cerebellar vasculitis and necrosis (WSC 2014, Conf 21, Case 4), granulomatous lymphadenitis and hepatitis (WSC 2013 Conf 25 Case1) and tubulointerstitial nephritis (WSC 2011, Conference 7, Case 3.)
Vasculitis is a common lesion in association with PCV-2 infection. Porcine dermatitis and nephritis syndrome is characterized by a necrotizing and neutrophilic vasculitis of arterioles and capillaries of the skin and kidney (to include glomerular capillaries). Fibrinoid necrosis of septal capillaries within the lung and resultant focal alveolar hemorrhage and edema has been widely reported in the US and Europe.7 Necrotizing vasculitis within the cerebellum has been documented in porcine multisystemic wasting disease. Finally, cases of myocarditis in piglets are often associated with lymphohistiocytic coronary arteritis and periarteritis.7,8
The presumed pathogenesis of vasculitis in various forms of PCVAD is a Type III hypersensitivity reaction. In Type III hypersentivity, systemic disease results from the formation of antibodies to a protein (in this case, viral protein) with deposition of antigen-antibody complexes within the walls of vessels. Vessels in which blood is filtered to form other bodily fluids, such as urine and synovial fluid, are most affected (resulting in glomerulonephritis and polyarthritis respectively). Anitbodies (IgG and IgM) which have the ability to bind complement or bind to Fc receptors on leukocytes are the most likely to result in vascular lesions of fibrinoid necrosis. Analysis of damaged vessels may demonstrate the presence of immune complexes or complement, although levels in vessels outside the kidney may be low. PCV-2 antigen has been demonstrated within the walls of damaged blood vessels.
The effects of PCV-2 on vessels remains to be elucidated, but previous studies have shone light on some of its effects. PCV-2 antigen has been demonstrated in endothelial and inflammatory cells within the walls of vessels in both experimentally- and naturally-infected pigs. PCV-2 has the ability to infect endothelial cells, causing them to increase the expression of surface procoagulant activity. PCV-2 antigen has been identified in endothelial and inflammatory cells within necrotic vessels in infected pigs with granulomatous lymphadinitis as well as a peracute syndrome of acute pulmonary edema syndrome.8
References:
1. Allan G, Meehan B, Todd D, Kennedy S, McNeilly F, Ellis J, Clark E G, Harding J, Espuna E, Botner A, Charreyre C. Novel porcine Circoviruses from pigs with wasting disease syndromes. Vet Rec. 1998;142(17):467-468.
2. Allan GM, Ellis JA. Porcine
circoviruses: a review. J Vet Diagn Invest. 2000;12:3?14.
3. Clark EG. Post-weaning wasting
syndrome. Proc Am Ass Swine Prac. 1997;28:499?501.
4. Grierson SS, King DP, Sandvik
T, Hicks D, Spencer Y, Drew TW, Banks M. Detection and genetic typing of type 2
porcine circoviruses in archived pig tissues from the UK. Arch Virol.
2004;149(6):1171?1183.
5. Higgins RJ.
Glomerulo-nephropathy syndrome. Pig Vet J. 1993;31:160?163.
6. Jacobsen B, Krueger L, Seeliger F, Bruegmann M, Segalés J, Baumgaertner W. Retrospective study on the occurrence of porcine circovirus 2 infection and associated entities in Northern Germany. Vet Microbiol. 2009;138(1-2):27-33.
7. Kumar V, Abbas AK, Aster JC.
Diseases of the immune system. In: Robbins and Cotran Pathologic Basis
of Disease, 9th ed. Philadelphia, PA, Elsevier Saunders, 2015.
8. Opriessnig T, Meng XJ, Halbur
PG. Porcine circovirus type 2 associated disease (PCVAD): Update on current
terminology, clinical manifestations, pathogenesis, diagnosis, and intervention
strategies. J Vet Diagn Invest. 2007;19:591-615.
9. Opriessnig T, Langohr I.
Current state of knowledge on porcine circovirus type 2-associated lesions. Vet Pathol. 2013;50(1):23-38.
10. Rosell C, Segalés J,
Ramos-Vara JA, Folch JM, Rodriguez-Arrioja GM, Duran CO, Balasch M, Plana-Durán
J, Domingo M. Identification of porcine circovirus in tissues of pigs with
porcine dermatitis and nephropathy syndrome. Vet Rec. 2000;146:40-43.
11. Tischer I, Rasch R,
Tochtermann G. Characterization of papovavirus- and picornavirus-like particles
in permanent pig kidney cell lines. Zbl Bakt Hyg. 1974:A 226:153-167.
12. Tischer I, Gelderblom H,
Vettermann W, Koch MA. A very small porcine virus with a circular
single-stranded DNA. Nature. 1982;295:64-66.
13. Truyen U. [Familie Circoviridae. In: Selbitz H-J, Truyen U, Peter V-W, eds. Tiermedizinische Mikrobiologie, Infektions- und Seuchenlehre. 9. Auflage. Stuttgart: Enke-Verlag]. 2011;487-492.