Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 23
24 April, 2019
CASE II: 2014910070 (JPC 4066543)
Signalment: 8 year-8 month-old, spayed female, Italian gray hound, dog. (Canis familiaris)
History: The dog was presented to the veterinary clinic with skin rashes in inner thigh region. She was treated with antibiotic and antihistamine for a week. A week later, a few millimeters multiple hematomas and papules were observed from inner thigh, abdomen to neck. She was treated with another antibiotic, antiplasmin and steroid, but these lesions were not improved. Three weeks later, a skin in the neck including papules was biopsied, and submitted to pathological examination.
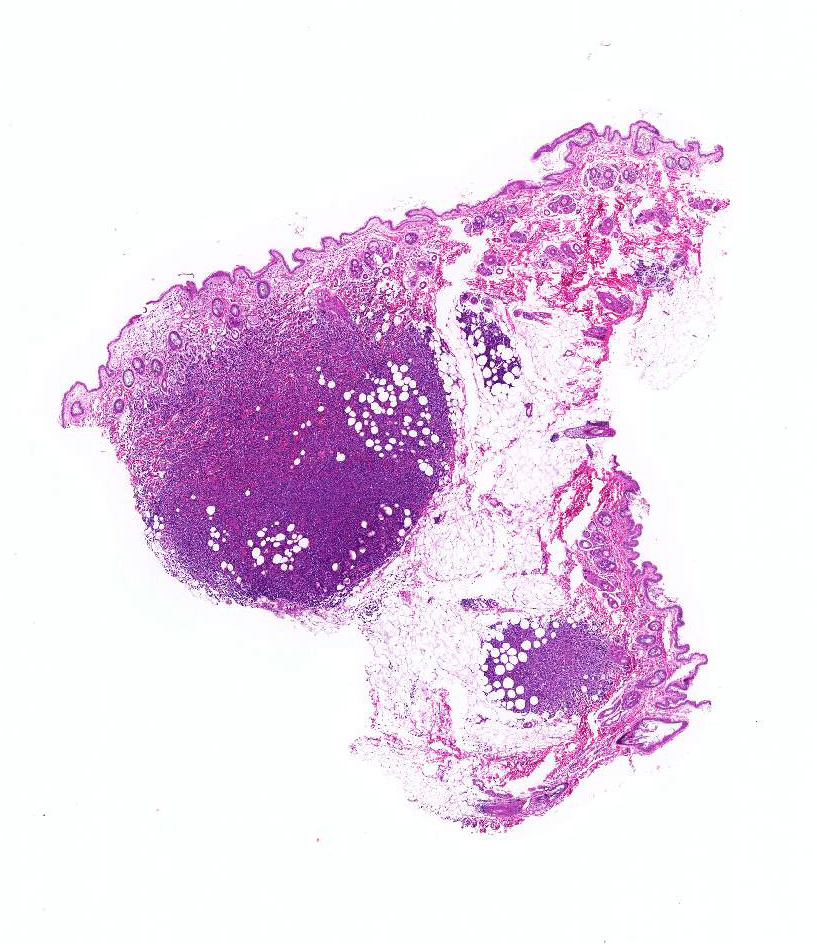
Gross Pathology: The cut surface of the papule after formalin fixation was brown to dark red.
Laboratory results: B lymphocyte monoclonality is detected by lymphocyte clonality analysis.
Microscopic Description:
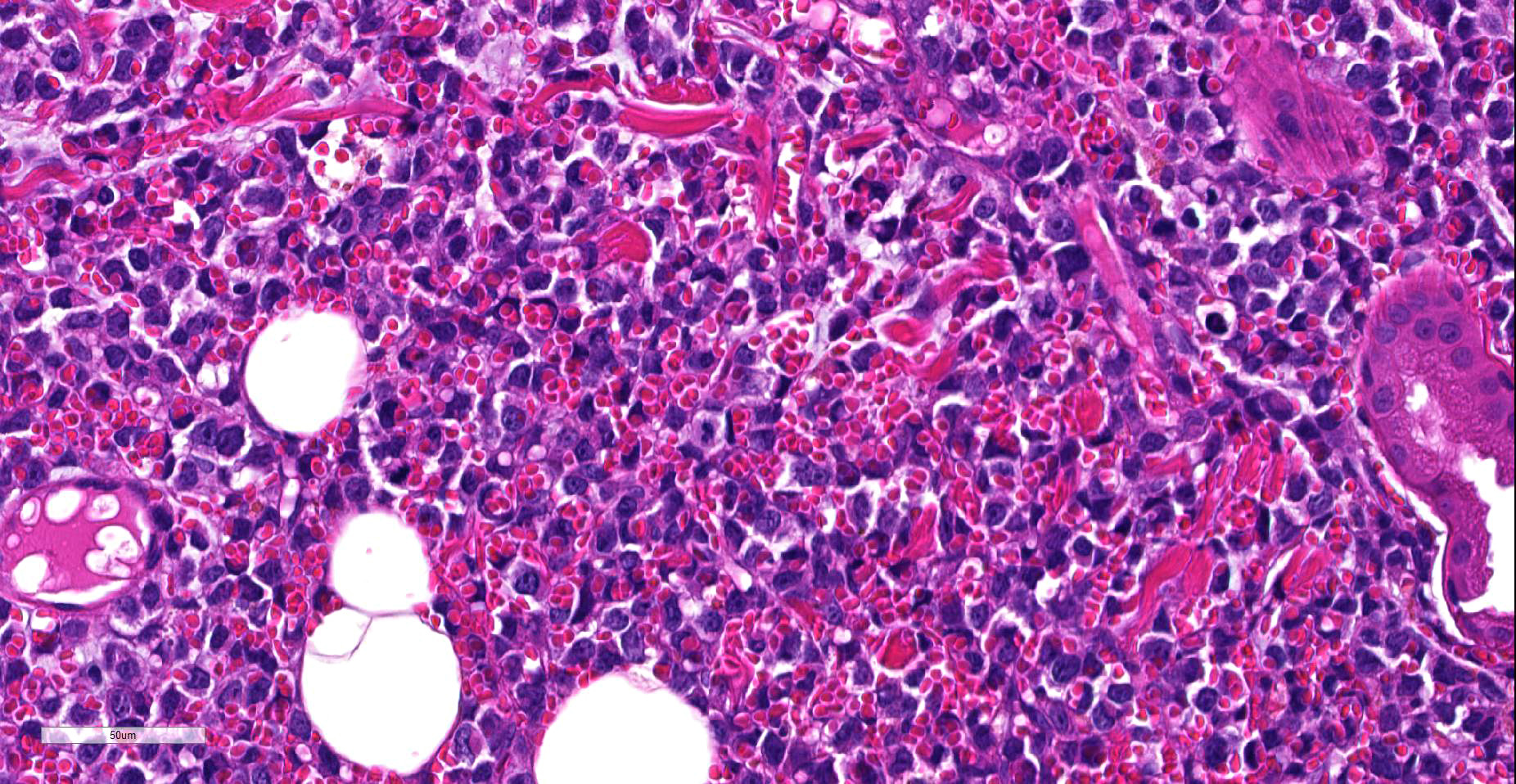
Histologically, a papule is composed of multiple foci. Monomorphic round tumor cells diffusely proliferate in dermis to subcutis of each focus Tumor cells do not have epitheliotropism. Tumor cells have oval to vesicular nuclei of intermediate-large size and scant to moderate amount of cytoplasm. One small to large nucleolus and finely distributed chromatin contain in the nucleus. Giant and multiple nucleus are occasionally seen. Mitotic figures are often observed. Mild hemorrhage is seen between tumor cells, and tumor cells frequently engulf red blood cells in the cytoplasm.
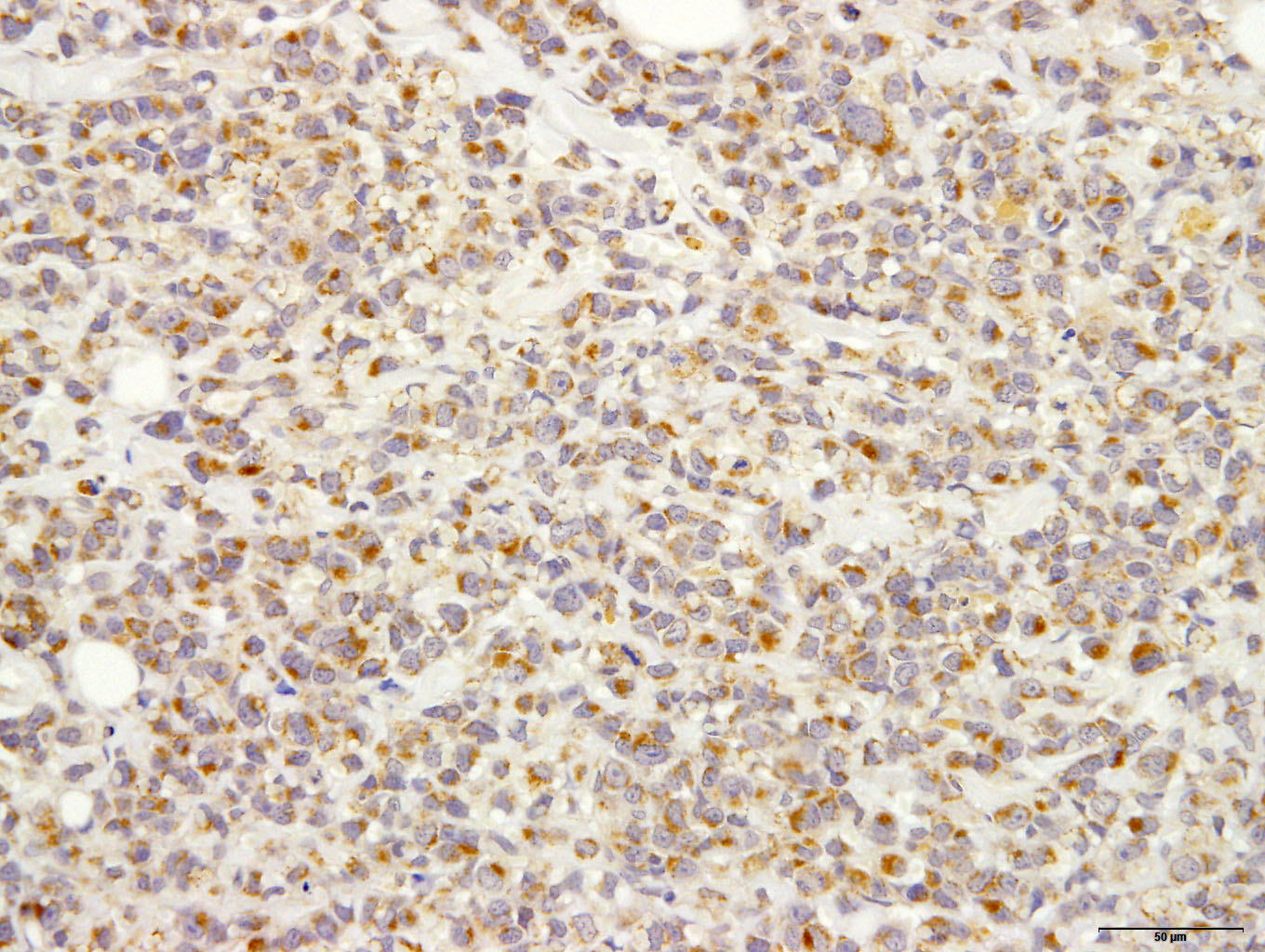
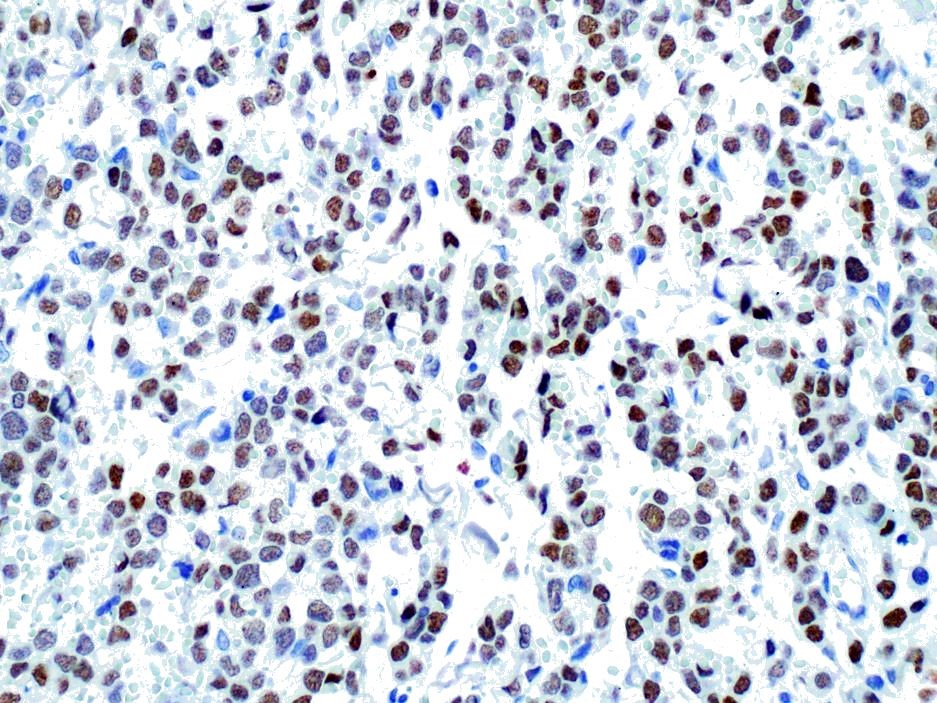
Immunohistochemically, almost all tumor cells are positive for CD20, lambda light chains Multiple myeloma Oncogene 1, weakly positive for CD79a but negative for CD3, Kappa light chains, and Iba-1.
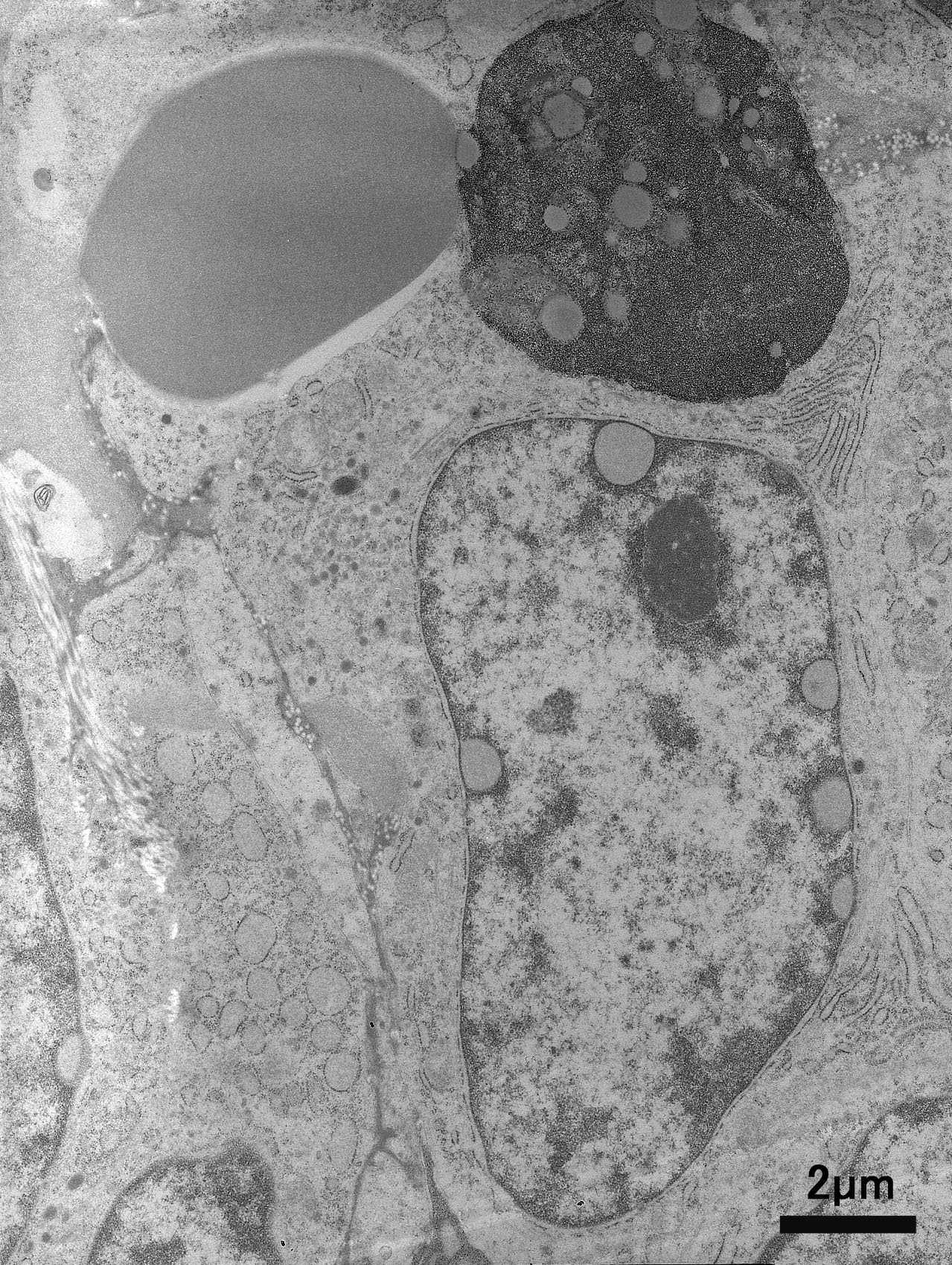
Ultrastructurally, tumor cells have nucleus with heterochromatin clumped under the nuclear membrane. Cytoplasm contain large amount of rough endoplasmic reticulum , but Golgi complex does not develop. Erythrocytes are taken into tumor cell cytoplasm . Primary and secondary lysosomes are often observed in the cytoplasm.
Contributor’s Morphologic Diagnoses:
Skin: hemophagocytic cutaneous lymphoma (B cell lymphoma), canine (Canis familiaris)
Contributor’s Comment: The present case was a round cell tumor with a characteristic of erythrophagocytosis. In animal, erythrophagia was reported most frequently in tumors of histiocytic cell origin6,10,14,15,17. Thus, the primary differential diagnosis was histiocytic sarcoma. However, tumor cells often had scant cytoplasm, and showed no immunohistochemical reactivity for Iba-1 (marker of histiocytic cell).
?In animals, erythrophagocytic tumors have been reported several tumors other than histiocytic sarcoma, including angiosarcoma, osteosarcoma, lymphoma, mast cell tumor, multiple myeloma and plasmacytoma1-3,12,13,19.
?In present case, tumor cells did not have nuclei with a cartwheel appearance and perinuclear halo (clear zone) in cytoplasm - thus, the cellar morphology was not similar to mature plasma cell. However, tumor cells had immunoreaction for CD79a, lambda light chain and MUM-1. MUM-1 was expressed more than 94% of canine plasmacytomas and few canine B cell lymphoma16. Developed rough endoplasmic reticulum like plasma cell was seen in the cytoplasm by ultrastructural observation. It was suggested tumor cells had partially plasmacytic differentiation.
Lymphoma with plasmacytic differentiation included plasmacytoma, lymphoplasmacytic lymphoma and plasmablastic lymphoma. We compared the cellular morphology and immunohistochemical reaction between present case and those tumors with plasmacytic differentiation (Table 1).
?Typical plasmacytomas are composed of small to medium size round cells. The cells are characterized by moderate to slightly abundant cytoplasm with an eosinophilic or amphophilic appearance. Nuclei are round to oval, small and irregular with single or multiple nucleoli and large, dense chromatin. Mitotic activity was varied but was usually low. The tumor cells are immunoreactive for CD79a, lambda and kappa light chains, MUM-17,16,18 and .in a few case are positive for CD2016.
?Lymphoplasmacytic lymphoma is composed of small lymphocytes of a plasmacytoid type. The cells have characteristics of chronic lymphocyte lymphoma or lymphocytic lymphoma of intermediate type. The cells have small (slightly larger than erythrocyte) round nuclei; numerous small chromocenters, absent or small nucleoli and slight larger than scant cytoplasm. Rare immunoblasts are often present. The mitoses were very low. The tumor cells were immunoreactive for CD79a9,18.
?Plasmablastic lymphoma is a variant of diffuse large B-cell lymphoma, characterized by morphology and immunohistochemical criteria of plasmacytic differentiation. The cells are round or oval, of medium to large size with immunoblastic/centroblastic morphologic features or classic “plasmablastic” morphologic features, with scant to abundant cytoplasm, and a prominent central nucleolus or multiple smaller nuclei with chromatin clumped under the nuclear membrane. The mitoses were frequent. The cells do not express B cell makers such as CD20 and CD79a, but express of plasmacyte markers as MUM-14,8.
?The present case has a cellar morphology similar to plasmablastic lymphoma, but differed from it by immunohistochemical features. Thus, the present case was diagnosed as B cell lymphoma.
Canine cutaneous lymphoma is reported in about 3-8% of all canine lymphoma, and is histologically divided into epitheliotropic and non-epitheliotropic lymphoma5,7. The majority of canine cutaneous non-epitheliotrophic lymphomas are of T cell origin, B- cell origin is rare 5,7.
?Animals with erythrophagocytic neoplasms in the spleen and liver often have non-regenerative anemia 6,10-12,15,17. In the present case, as the affected animal did not show obvious anemia at the time of a biopsy, it was suggested that our lymphoma may localized in the skin.
Contributing Institution:
Laboratory of Pathology, Faculty of Pharmaceutical Sciences, Setsunan University,
45-1 Nagaotohge-cho, Hirakata, Osaka 573-0101, Japan
http://www.setsunan.ac.jp/~p-byori/
JPC Diagnosis: Haired skin: Plasmablastic lymphoma, large cell, high grade, plasmablastic, with marked erythrophagocytosis
JPC Comment:
Conference participants agreed that the morphology of this unique neoplasm is that of a large cell (neoplastic cells exceed 2x that of an erythrocyte), high grade neoplasm (based on a high mitotic count) but further identification of the cells on HE is difficult, especially given the degree of erythrocytosis exhibited by these cells. Conference participants identify significant plasmacytic differentiation among the neoplastic cells, although mature plasma cells were scattered throughout the neoplasm, as evidenced both on the HE stain and several immunostains run at the JPC (plasma cells commonly pick up immunostains in a non-specific fashion)
The CD-20 immunostain was repeated at the JPC and neoplastic cells was strongly positive; and a MUM-1 stain performed at UPenn was also positive. An IBA-1 stain demonstrated numerous infiltrating histiocytes. IBA-1 demonstrated some histiocytes within the neoplasm, but not a large component. Based on the immunohistochemical findings in this case, the moderator assessed this neoplasm as a likely B-cell neoplasm with early plasmacytic differentiation.
While we have seen cutaneous B-cell lymphoma in the skin numerous times, the erythrophagocytosis seen in this case is unique and remarkable. A Pubmed review of erythrophagocytic forms of lymphoma noted several cases of T-cell neoplasm exhibiting eythrophagocytosis in both humans and animals, this case appears unique for its B-cell erythophagocytic activity. As B-cells do not have intracellular machinery to break down erythrocytes into hemosiderin and lipofuscin like macrophages, this tumor is especially striking in the presence of apparently viable erythrocytes and a lack of hemosiderin pigment.
The moderator commented that while the presence of erythrocytes within neoplastic cells is unusual, it is prudent to believe in the conventional wisdome that neoplastic cells of any lineage may engulf erythrocytes if they desire to do so.
References:
- Barger AM, Skowronski MC, MacNeill AL. Cytologic identification of erythrophagocytic neoplasms in dogs. Vet Clin Pathol.2012;41:587-589.
- Bertazzolo W, Dell'Orco M, Bonfanti U, et al. Canine angiosarcoma: cytologic, histologic, and immunohistochemical correlations. Vet Clin Pathol. 2005;34:28-34.
- Carter JE, Tarigo JL, Vernau W, Cecere TE, Hovis RL, Suter SE: Erythrophagocytic low-grade extranodal T-cell lymphoma in a cat. Vet Clin Pathol. 2008;37:416-421.
- Castillo JJ, Reagan JL. Plasmablastic lymphoma: a systematic review. Scientific World Journal. 2011; 11:687-696.
- Day MJ. Immunophenotypic characterization of cutaneous lymphoid neoplasia in the dog and cat. J Comp Pathol. 1995;112:79-96.
- Friedrichs KR, Young KM. Histiocytic sarcoma of macrophage origin in a cat: case report with a literature review of feline histiocytic malignancies and comparison with canine hemophagocytic histiocytic sarcoma. Vet Clin Pathol. 2008;37:121-128.
- Gross TL IP, Walder EJ, Affolter VK. Lymphocytic tumors.In: Skin diseases of the dog and cat. 2nd ed. Oxford, UK: Blackwell; 2005:866-893.
- Hsi ED, Lorsbach RB, Fend F, Dogan A: Plasmablastic lymphoma and related disorders. Am J Clin Pathol. 2011;136:183-194.
- Jaffe VE, Harris, N.L, Stein, H, Varidiman, J. W: Pathology &Genetic Tumor of Hematopoietic and Lymphoid Tissues. In: World Health Organization Classification of tumours. Lyon, France: 2001:120-134,171-174.
- Kraje AC, Patton CS, Edwards DF. Malignant histiocytosis in 3 cats. J Vet Intern Med. 2001;15:252-256.
- Ledieu D, Palazzi X, Marchal T, Fournel-Fleury C. Acute megakaryoblastic leukemia with erythrophagocytosis and thrombosis in a dog. Vet Clin Pathol. 2005;34:52-56.
- Madewell BR, Gunn C, Gribble DH: Mast cell phagocytosis of red blood cells in a cat. Vet Pathol. 1983;20:638-640.
- Marks SL, Moore PF, Taylor DW, Munn RJ. Nonsecretory multiple myeloma in a dog: immunohistologic and ultrastructural observations. J Vet Intern Med. 1995;9:50-54.
- Matsuda K, Nomoto H, Kawamura Y, Someya Y, Koiwa M, Taniyama H. Hemophagocytic histiocytic sarcoma in a Japanese black cow. Vet Pathol.2010;47:339-342.
- Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol.2006;43:632-645.
- Ramos-Vara JA, Miller MA, Valli VE. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: comparison with CD79a and CD20. Vet Pathol. 2007;44:875-884.
- Rossi S, Gelain ME, Comazzi S. Disseminated histiocytic sarcoma with peripheral blood involvement in a Bernese Mountain dog. Vet Clin Pathol. 2009;38:126-130.
- Valli VE, Jacobs RM, Parodi, AL, Vernau W, Moore, P.F. Hematopoietic Tumor of Domestic Animals. vol. ?. Wasington DC, USA: World Healt Organization International Histological Classfication of tumors of domestic animals; 2002:28-38.
- Yearley JH, Stanton C, Olivry T, Dean GA. Phagocytic plasmacytoma in a dog. Vet Clin Pathol. 2007;36:293-296.