Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 3
Sept 19, 2018
CASE IV: 13-555-21 (JPC 4034756-00).
Signalment: 8-month-old, neutered male Airedale, Canis familiaris, canine.
History: Presented with depression, vomiting and diarrhea. Significantly raised urea, creatinine, phosphate, sodium, calcium. Did not improve with three days of intravenous fluid therapy and was euthanised. Only the kidneys were sent by the clinician via the surgical biopsy service.
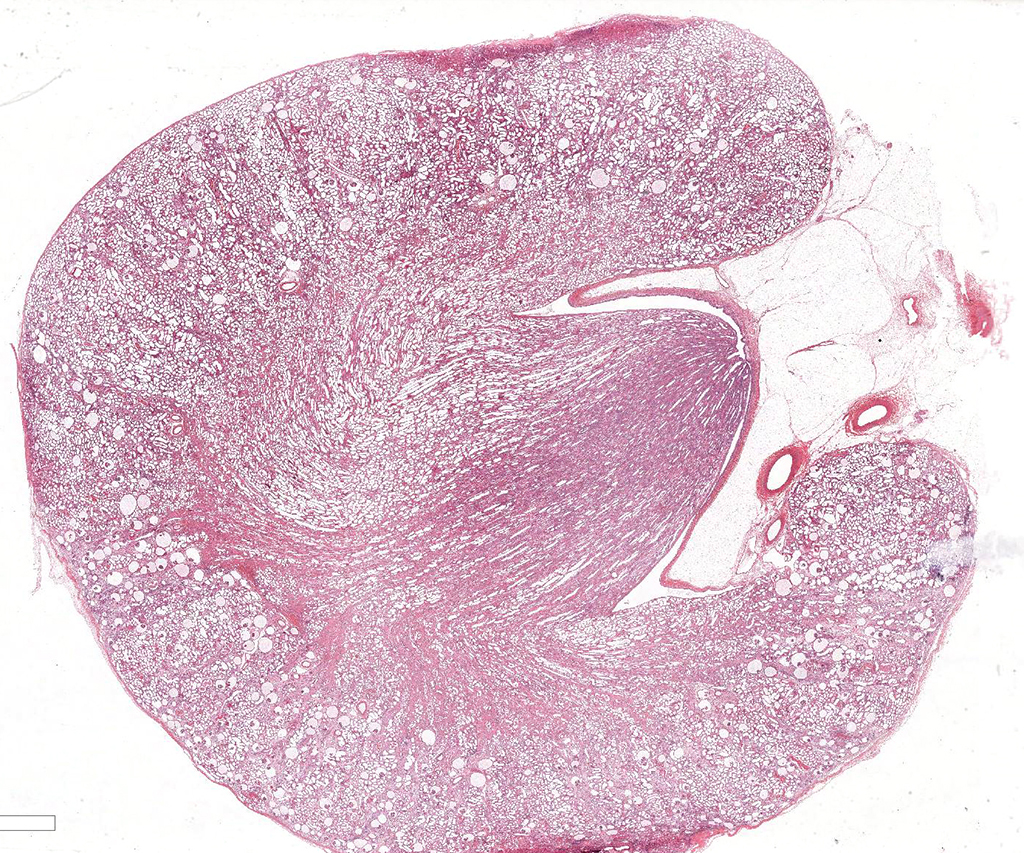
Gross Pathology: Irregularly pitted with an undulating capsular surface.
Laboratory results: None.
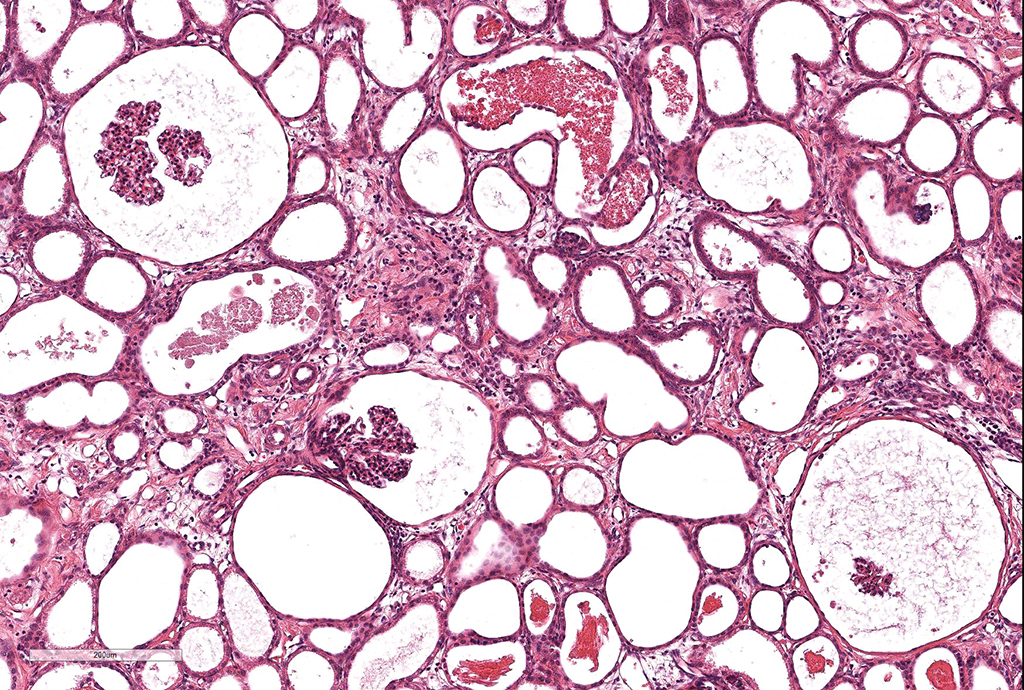
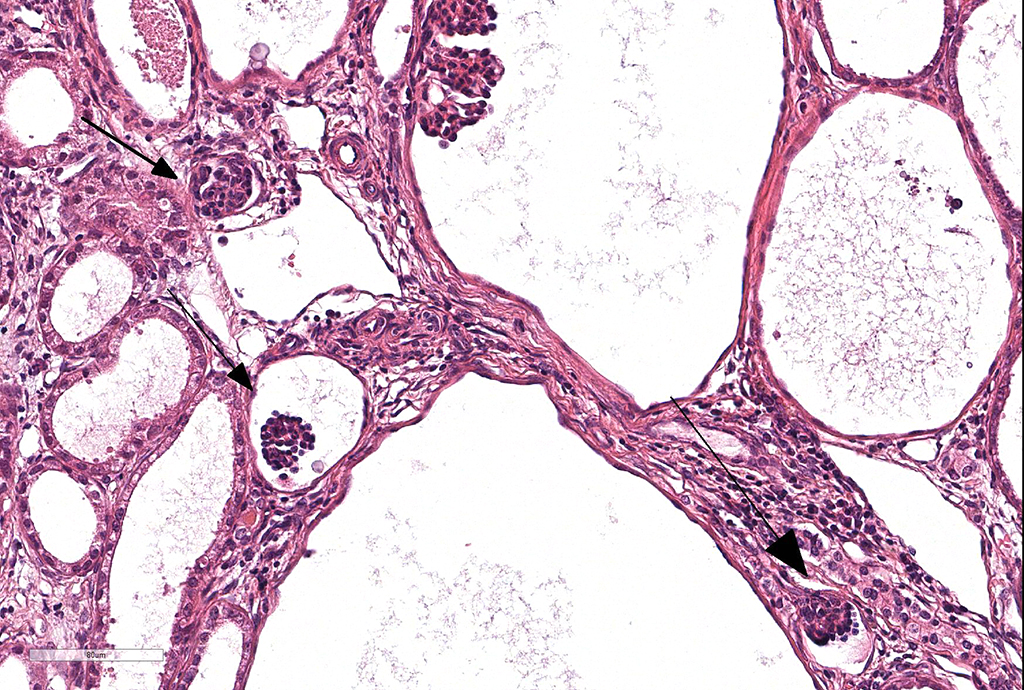
Microscopic Description: Kidney. Diffusely, the renal capsule is moderately thickened and multifocally depressed. The subjacent parenchyma consists of irregular foci of loose primitive stroma that contains small caliber vessels, primitive tubules and tubules lined by irregularly tall
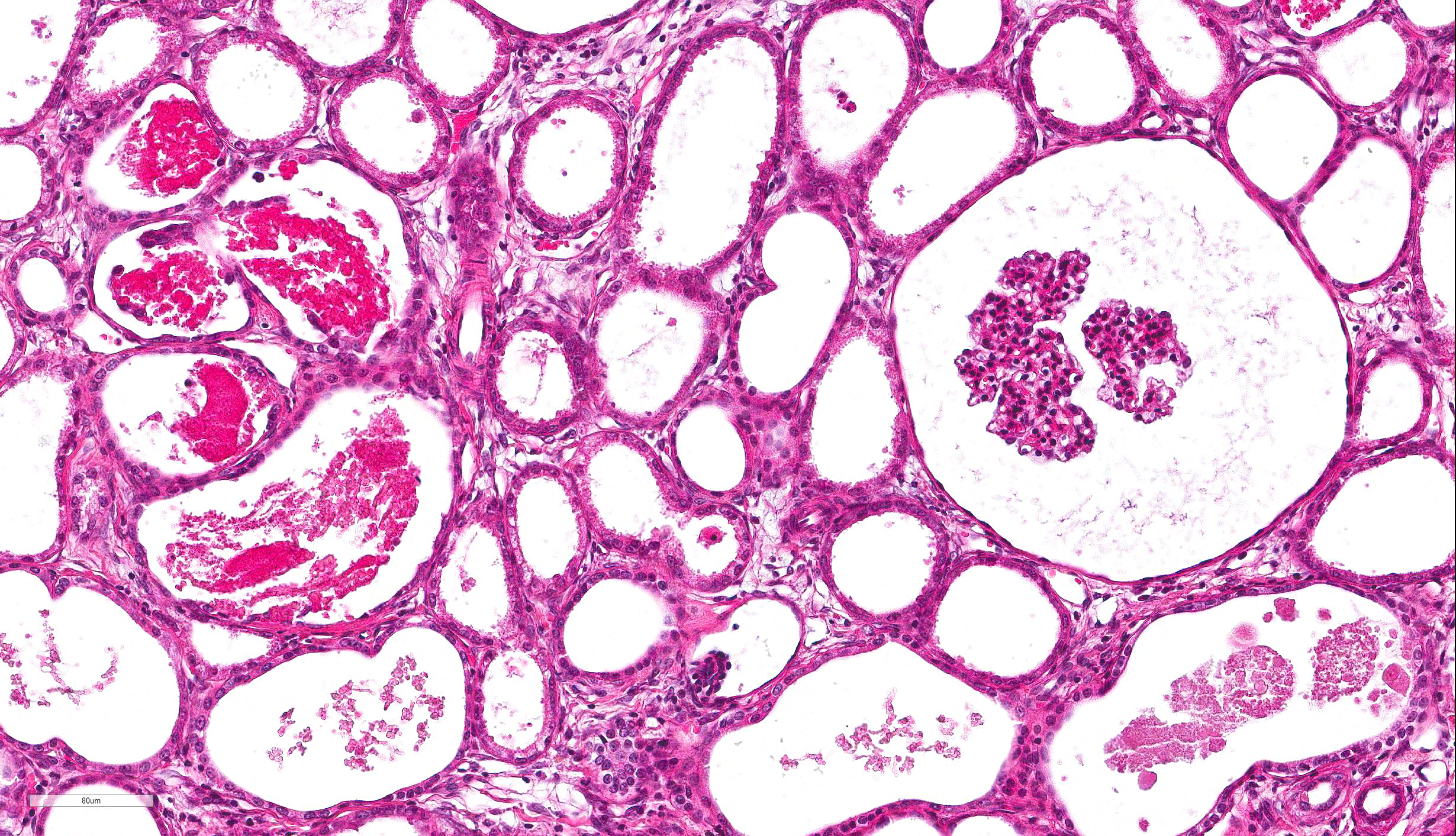
columnar epithelium with basilar nuclear location (persistent metanephric ducts). Extending radially from the outer cortex to the medulla are multifocally extensive areas of loose fibrous stroma (interstitial fibrosis; confirmed with trichrome staining). Predominantly within these areas and affecting over 80% of glomeruli, Bowman's spaces are markedly dilated and multifocally contain a moderately shrunken glomerulus. Multifocally within the superficial cortex glomeruli are small with peripheral nuclei and indistinct capillary tufts (fetal glomeruli). Multifocally Bowman's capsules contain intramural, spherical (2-15μm diameter) basophilic material (mineral; confirmed with von Kossa staining). Diffusely cortical tubules are markedly dilated (up to 120μm in diameter). Tubules are multifocally lined either by plump cuboidal to flattened
(attenuated) tubular epithelium or epithelium that is enlarged and hypereosinophilic (tubular degeneration) or multifocally by enlarged, severely vacuolated epithelium with karyorrhexis and karyolysis (tubular necrosis). Multifocally tubules contain amorphous hyaline to granular hypereosinophilic material (protein casts) and tubular epithelium is multifocally replaced or deviated by amorphous basophilic material (mineral; confirmed with Von Kossa staining). Collecting ducts range in diameter from 20 to 140μm and are variously lined by attenuated to plump cuboidal and columnar epithelium, which multifocally partially occludes the lumen (atypical epithelium). Multifocally collecting ducts are surrounded by areas of loose, undifferentiated mesenchyme that is focally infiltrated by low numbers of neutrophils; multifocally lumina contain sloughed pyknotic and karyorrhectic debris (necrosis). The cortical interstitium is infiltrated by low numbers of lymphocytes and plasma cells.
Contributor's Morphologic Diagnoses: 1. Kidney; dysplasia with fetal glomeruli, persistent metanephric ducts, primitive mesenchyme and atypical tubular epithelium.
- Kidney; marked diffuse tubular and Bowman's space ectasia with multifocal tubular degeneration, necrosis and mineralization and moderate interstitial fibrosis
- Kidney: Tubular loss, diffuse, moderate, with granular cast formation and rare epithelial necrosis.
Contributor’s Comment:
Canine renal dysplasia is usually congenital and often thought to be hereditary resulting in disorganized development of renal parenchyma due to anomalous differentiation.4 It has been reported in many breeds including the Golden Retriever, Beagle, Dutch Kookier, Miniature Schnauzer, Shih Tzu, Lhasa Apso, Great Dane, Samoyed, Alaskan Malamute, Cavalier King Charles Spaniel and Bulldog. 1,2,3,5,9,11
Disease in the early neonatal period may also cause renal dysplasia by affecting incompletely differentiated renal tissue. This has been reported in puppies that survived intraperitoneal infection with canine herpesvirus at 2 days of age and were sacrificed at 11 and 16 days post infection.8 Similarly renal dysplasia has been reported in calves and kittens following infection with bovine pestivirus and feline parvovirus, respectively.4
The kidney develops from successive structures, which overlap in their formation. Renal malformation can therefore occur in many patterns. The developing structures include the pronephros, mesonephros and ultimately, the metanephros. The pronephros and mesonephros degenerate to vestigial remnants but are crucial in the correct formation of the metanephros. The metanephros or 'definitive kidney' forms from complex interaction of the ureteral bud and metanephric blastema. This may explain why malformations of the kidney are often accompanied by ureteral anomalies in man, although this is not commonly reported in dogs.4, 10
The clinical presentation and the age at which signs develop are variable. Renal dysplasia in neonates has been associated with reduced appetite, intermittent vomiting, dullness, poor growth and polyuria/polydipsia. Puppies may not survive to weaning; longer survival (over 4 months) results in clinical signs that can be attributed to uremia such as vomiting, diarrhea, anemia, nervous signs and fibrous osteodystrophy ("rubber jaw").6
Renal dysplasia may be uni- or bilateral. Grossly, affected kidneys may be small and therefore potentially they could be misdiagnosed as hypoplastic. They may also be misshapen, lobulated, contain an irregularly thin cortex or thick walled cysts and they may be associated with dilated tortuous ureters.4,9 They can be indistinguishable from end stage renal lesions in old dogs.9 Alternatively, they may appear grossly normal, and therefore histopathological examination is required for diagnosis.4
In the largest case series available, 45 dogs of various breeds were diagnosed with renal dysplasia. Histological features were described as primary, compensatory and degenerative or inflammatory. At least one of the following primary features was identified in each case:
1) Asynchronous differentiation of nephrons, defined by the presence of fetal or
immature glomeruli and/or tubules (40/45 cases).
2) Persistent mesenchyme: a loose stroma found in the medulla, which is alcian blue
positive and trichrome stain negative (25/45 cases).
3) Atypical tubular epithelium reported as adenomatoid cuboidal or clusters of
squamous epithelial cells (7/45 cases).
4) Persistent metanephric ducts, which are ducts in the outer medulla lined by tall
pseudostratified columnar epithelium and often dilated (6/45 cases).
5) Dysontogenic metaplasia: represented as cartilaginous or osseous metaplasia (2/45
cases).7
There is disagreement between authors on the features required for a diagnosis of renal dysplasia. Some authors believe juvenile nephropathy or juvenile renal disease is a more appropriate term for cases of renal dysplasia which do not exhibit primitive ducts or dysontogenic metaplasia. Dysontogenic metaplasia and primitive metanephric ducts are the least commonly observed features in 'renal dysplasia', therefore making the classification restrictive and excluding many cases where fetal glomeruli alone are observed. Juvenile nephropathy is a general term that includes non-inflammatory, degenerative, developmental and chronic renal disease of unknown pathogenesis and is therefore non-specific. However this may be a more appropriate term for cases whereby the pathogenesis of the dysplasia is unknown or unclear. Further increasing the confusion, 'familial renal disease' has been used interchangeably with renal dysplasia in the literature. 4,5,9
Compensatory features were described in 20/45 cases and included enlarged glomeruli with mesangial hyperplasia and cortical tubular dilation with hypertrophic and hyperplastic cuboidal epithelial lining. Degeneration and inflammatory changes may be severe and therefore obscure primary features required for diagnosis.
Degenerative/inflammatory features were present in all cases and predominantly included interstitial fibrosis, which was often segmental and within areas of fetal glomeruli. The appearance of tubulointerstitial nephritis and pyelonephritis was in parallel with the severity of fibrosis. Other changes observed included dystrophic mineralization, cystic glomerular atrophy, microcystic tubules, retention cysts and glomerular lipidosis.8
JPC Diagnosis: 1. Kidney: Asynchronous maturation, diffuse, marked, with glomerulocystic atrophy, fetal glomeruli, marked tubular ectasia, and mild chronic interstitial nephritis.
Conference Comment: The contributor has provided a concise but relatively thorough discussion of renal dysplasia in the dog.
A slightly more detailed review of renal embryogenesis may help in understanding its development. During fetal development, the ureteric bud, an evagination of the mesonephric duct, develops into the metanephros. The ureteric bud extends cranial dorsally into the overlying metanephric blastema whereupon successive divisions of the ureteric bud result in formation of the collecting ducts. Interaction with mesenchymal cells within the metanephric blastoma results of the formation of the remainder of the nephron, including glomeruli and tubules. The structures initially form at the cortical medullary junction, but as the collecting ducts elongate, or subsequently formed in the superior aspects of the cortex. The first few weeks of life see additional development of renal structures.9
As the importance of the ureteric bud in the overall development of the kidney now becomes clear, it can be understood how ureteral abnormalities during development have such a profound impact on the developing kidney. Abnormal placement of the ureters during development as well as lower urinary tract obstruction have both been identified as major contributors to renal dysplasia in humans.7, 10 Such extra-renal abnormalities are not commonly reported in dogs, but this may be the result of incomplete autopsy examination of the entire urinary tract.9
As renal dysplasia is a complex, multifactorial disease, obviously there are many other contributors than simply the placement of the ureteric bud. Regulation of “renal branching morphogenesis” is controlled by a wide range of transcription factors, growth factors, and cell surface signaling peptides which, if deficient, results in improper induction of the metanephric blastema and ultimately, renal dysplasia.4 Subsequent to the observation that cyclooxygenase-2 (Cox-2) deficient mice exhibit renal changes similar to renal dysplasia in dogs, the Cox-2 genes of Lhasa Apso affected with familiar renal dysplasia were sequenced and compared to that of normal dogs.12 Small insertions and the deletions of GC boxes upstream of the ATG translation start site (which are putative SP1 transcription factor binding sites) were found upstream of the A translation start site in affected animals.12
During the slide description, the moderator commented on the difficulty on the precise identification of primitive mesenchyme and metanephric tubules in cases of juvenile nephropathy, as well as the spirited discussions that it often engenders among experts in renal disease. Without precise definitions for these terms even among experts, their identification in many cases is often quite subjective and fraught with peril. In this particular case, after some discussion, the “primitive mesenchyme” identified by some attendees was determined to be edematous mature collagen, and immature tubules more likely to be the result of interesting sectioning of collecting ducts.
The moderator also commented on the amount of debris within tubules which is uncommon in cases of juvenile dysplasia. In this case, this cellular debris is more suggestive of a second, more recent tubular insult, resulting in this animal’s acute uremic presentation. The developmental abnormalities seen in this kidney, although dramatic, likely would not have resulted in a death at 8 months on their own. She also commented on the profound ectasia of Bowman’s capsules in this case, strongly suggesting downstream obstruction (which is corroborated by the marked tubular ectasia also seen in this case.)
The term “renal dysplasia” itself appears to be falling from favor today among pathologists and clinicians, with other terms such as “juvenile nephropathy” and “renal maldevelopment” gaining favor.
Contributing Institution:
The Royal Veterinary College,
PPB, Hawkshead Campus,
Hawkshead Lane,
South Mymms,
AL9 7TA
www.rvc.ac.uk/pathology-and-diagnostic-laboratories
References:
- Bruder MC, Shoieb AM, Shirai N, Boucher GG, Brodie TA. Renal dysplasia in beagle dogs:
four cases. Toxicol Pathol. 2010; 38:1051-1057.
- Hoppe A, Swenson L, Jonsson L, Hedhammar A. Progressive nephropathy due to renal
- Kerlin RL, Van Winkle TJ. Renal dysplasia in golden retrievers. Vet Pathol. 1995; 32: 327-
- Maxie MG. Urinary system. In: Jubb, Kennedy, and Palmer's Pathology of Domestic
- Morton LD, Sanecki RK, Gordon DE, Sopiarz RL, Bell JS, Sakas PS. Juvenile renal disease in
- Nash AS. Familial renal disease in dogs. J Small Anim Pract 1989; 30:178-183.
- Nagata M, Sawako S, Shu Y. Pathogenesis of dysplastic kidney associated with urinary tract obstruction in utero. Nephr Dial Transplant 2002; 17(Suppl 9): 37-38.
- Percy DH, Carmichael LE, Albert DM, King JM, Jonas AM. Lesions in puppies surviving
- Picut CA, Lewis RM. Microscopic features of canine renal dysplasia. Vet Pathol
- Risdon RA. Renal dysplasia. J Clin Path 1971; 24:57-71.
- Schulze C, Meyer HP, Blok AL, Schipper K, Van Den lngh TS. Renal dysplasia in three
- Whitely MH, Bell JS, Rothman DA. Novel allelic variants in the canine cyclooxgenase-(Cox-2) promoter are associated with renal dysplasia in dogs. PLoS One 2011, 6(2) e16684.
dysplasia in shih tzu dogs in Sweden: a clinical pathological and genetic study. J Small
Anim Pract.1990; 31:83-91.
329.
Animals. 5th, ed. Philadelphia, PA: Elsevier Saunders; 2007; Vol 2, pp. 437-442
miniature schnauzer dogs. Vet Pathol 1990; 27:455-458.
infection with canine herpesvirus. Vet Pathol 1971; 8:37-53.
1987; 24:156-163.
young adult dutch kookier dogs. Vet Quart 1998; 20:146-148