Signalment:
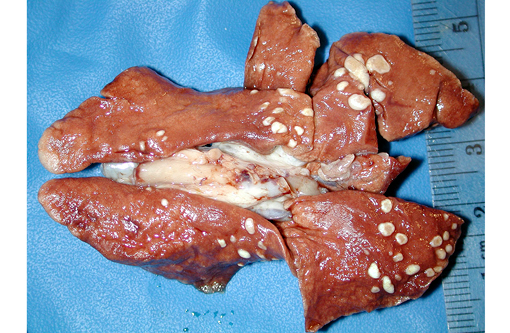
Gross Description:
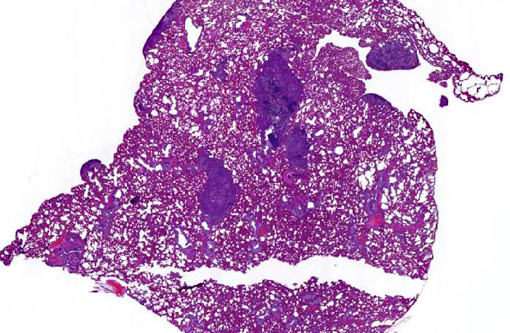
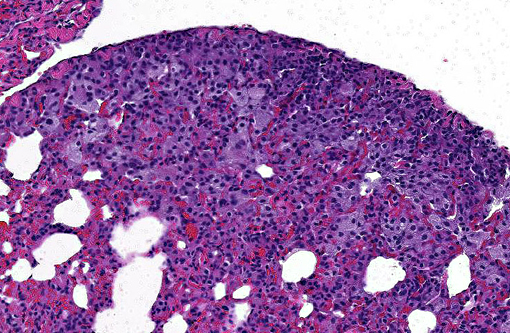
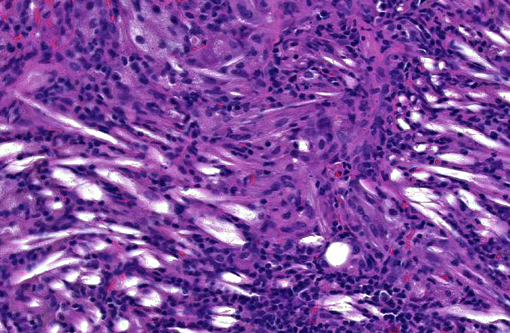
Histopathologic Description:
Multifocal, often coalescing areas of lipid pneumonia are present. These are often (correlative to the gross distribution of lesions seen) present in subpleural locations, although foci deep within pulmonary parenchyma are often noted. Some areas consist solely of homogeneous alveolar aggregates of foamy macrophages. Others are comprised of more dense mixed inflammatory infiltrates including histiocytes, lymphocytes, scattered neutrophils and occasional multinucleated giant cells. Cholesterol clefts and crystals are often seen in association with these latter areas. Infrequent focal areas of alveolar thickening and mild interstitial fibrosis are also noted in some lesions.
In general, most other adjacent lung tissue is atelectatic due to post-mortem collapse, but evidence of other concurrent chronic or active pulmonary disease processes (e.g. foreign body material, observable organisms, neoplasia, etc.) was not noted.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In most cases when present, EnLP is an incidental finding secondary to some (often prior & undetermined) direct pneumocyte injury or bronchial obstructive process, and effort should be made to detect either resolved or active concurrent pulmonary disease. However, caution should be exercised in ascribing a major primary role in morbidity and mortality to these lesions alone.
The cause of death in this animal was thought to be debilitation and dehydration secondary to ferret enteric coronavirus infection (as suggested by a typical clinical presentation(15) and confirmed by PCR evaluation of feces). Microscopic assessment of the GI tract was of little additional benefit due to the degree of autolytic change present. Although the clinical presentation and other gross and histopathologic findings are distinctly different, the lesions of EnLP should not be mistaken for those associated with multisystemic coronavirus infection (resembling FIP) in ferrets.(4)
JPC Diagnosis:
Conference Comment:
This case illustrates EnLPs classic histologic presentation as subpleural accumulations of histiocytes, lipid, and cholesterol. This distinctive subpleural location is curious given the proposed pathogenesis of pneumocyte damage and subsequent cholesterol release from disintegrating cell membranes.(7) Conference participants could not determine how such alveolar accumulations routinely end up forming subpleural nodules, and most agree with the contributors suspicions of a multifactorial pathogenesis in the formation of EnLP.
References:
1. Banjar H. Lipoid Pneumonia: A Review. Bahrain Medical Bulletin 25 (1): 36-39 (2003)
2. Brown CC. Endogenous Lipid Pneumonia in Opossums from Louisiana. Journal of Wildlife Diseases 24(2): 214-219 (1988)
3. Dungworth D. The respiratory system. In: Jubb K, Kennedy P, Palmer N, eds. Pathology of domestic animals. 3rd ed. Orlando, Fla: Academic Press Inc, 1985; 470-474
4. Garner MM et al: Clinicopathologic Features of a Systemic Coronavirus-Associated Disease Resembling Feline Infectious Peritonitis in the Domestic (Mustela putorius). Vet Pathol 45:236-246 (2008)
5. Guarda F, Bollo E, Macchi E. Lipoid Pneumonia in the Red Fox (Vulpes vulpes). JME 4: 13-16 (1997)
6. Himsworth CH, Malek S, Saville K, Allen AL. Endogenous lipid pneumonia and what lies beneath. Can Vet J. 2008;49(8):813-815.
7. Jones DJ, Norris CR, Samii VF, Griffey SM: Endogenous lipid pneumonia in cats: 24 cases (1985-1998). JAVMA 216 (9): 1437-1440 (2000)
8. Lopez A. Respiratory system, mediastinum, and pleurae. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:530.
9. Marinez J, Reinacher M, Perpinan D, Ramis A: Identification of Group 1 Coronavirus Antigen in Multisystemic Granulomatous Lesions in Ferrets (mustela putorius furo). J Comp Path 138:54-58, 2008
10. Molina AM, Raya AI, Carrasco L, Blanco A, Monterde JG, Rueda A, Moyano R: Endogenous lipid pneumonia in rats after subacute exposure to CdCl2. Toxicology and Industrial Health 24: 677-681 (2008)
11. Personal communication, Dr. Bruce Williams
12. Raya AI, Fernandez-de Marco M, Nunez A, Afonso JC, Cortade LE, Carrasco L. Endogenous Lipid Pneumonia in a Dog. J Comp Path 135: 153-155 (2006)
13. Sharma A, Ohri S, Bambery P, Singh S: Idiopathic Endogenous Lipoid Pneumonia. Indian J Chest Dis Allied Sci 48: 143-145 (2006)
14. Weller W. Alveolar lipoproteinosis. In Respiratory system, monographs on pathology of laboratory animals, T.C. Jones, U. Mohr and RD Hunt (eds). Springer-Verlag, New York, New York, 171-176
15. Williams BH, Kiupel M, West KH, Raymond JT, Grant CK, Glickman LT: Coronavirus-associated epizootic catarrhal enteritis in ferrets. JAVMA 217 (4): 526-530 (2000)