Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 10
20 November 2019
CASE I: R17104D (JPC 4135946).
Signalment: Caribbean spiny lobster (Panulirus argus) of unknown sex and age
History: In 2016, 14 Caribbean spiny lobsters (Panulirus argus) were collected off of Summerland Key, Florida to supplement the resident population at a large public aquarium. The lobsters were transported and placed in quarantine at a separate facility. After 5 months, lobsters began showing clinical signs of lethargy and dying in the molt. Samples of hemolymph were submitted to the Louisiana Aquatic Diagnostic Laboratory (LADL) at Louisiana Animal Disease Diagnostic Laboratory (LADDL) and the University of Florida for PCR for White Spot Syndrome Virus (WSSV) and Panulirus argus Virus 1 (PaV1), respectively. Upon obtaining the PCR results, the remaining lobsters were euthanized and gross necropsies were performed on site at the quarantine facility. Formalin-fixed tissues were submitted to LADL for histopathological evaluation.
Gross Pathology: There were no significant gross lesions in the lobsters examined.
Laboratory results:
PCR for White Spot Syndrome Virus (WSSV): Negative
PCR for Panulirus argus Virus 1 (PaV1): Positive
Microscopic Description:
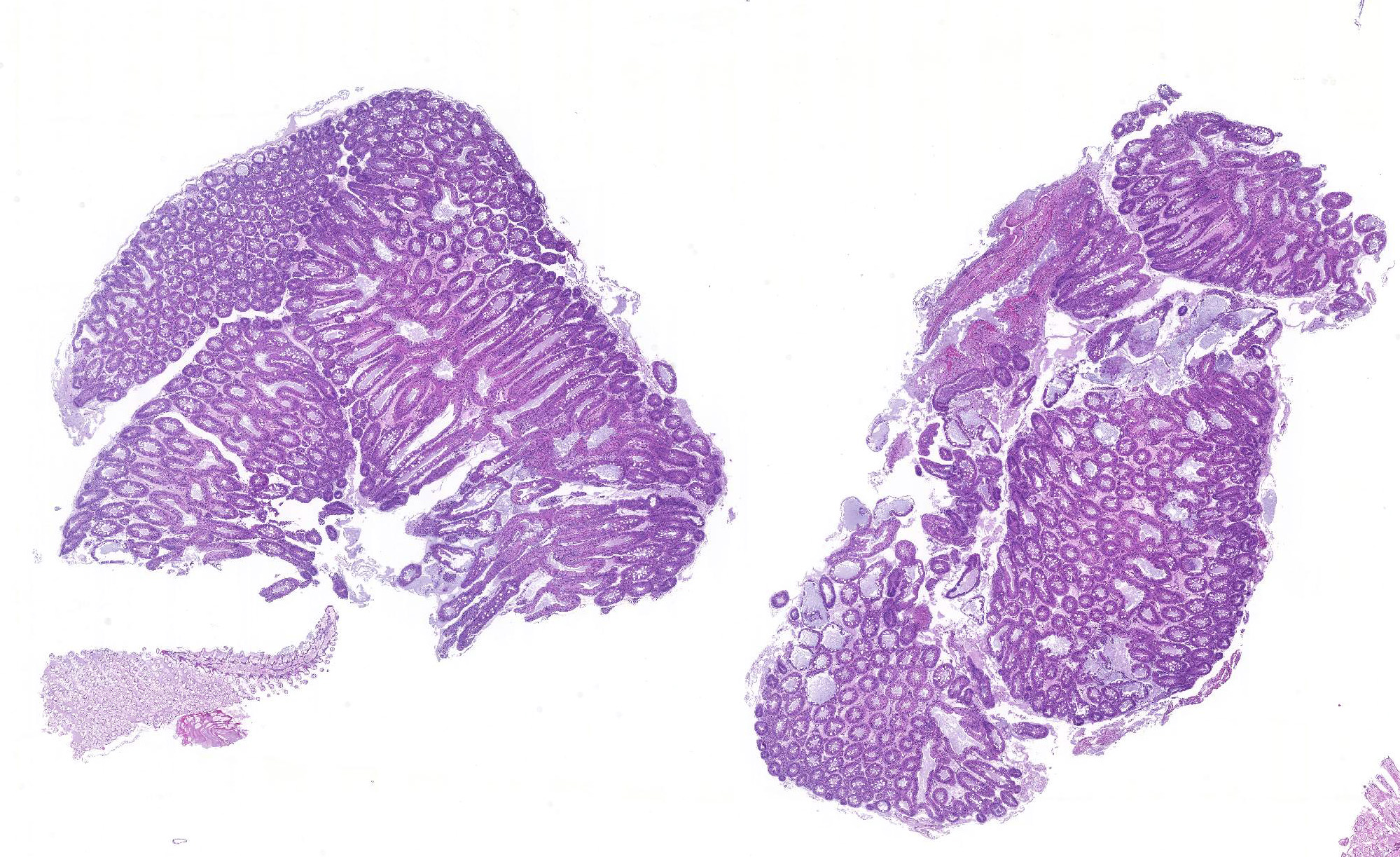
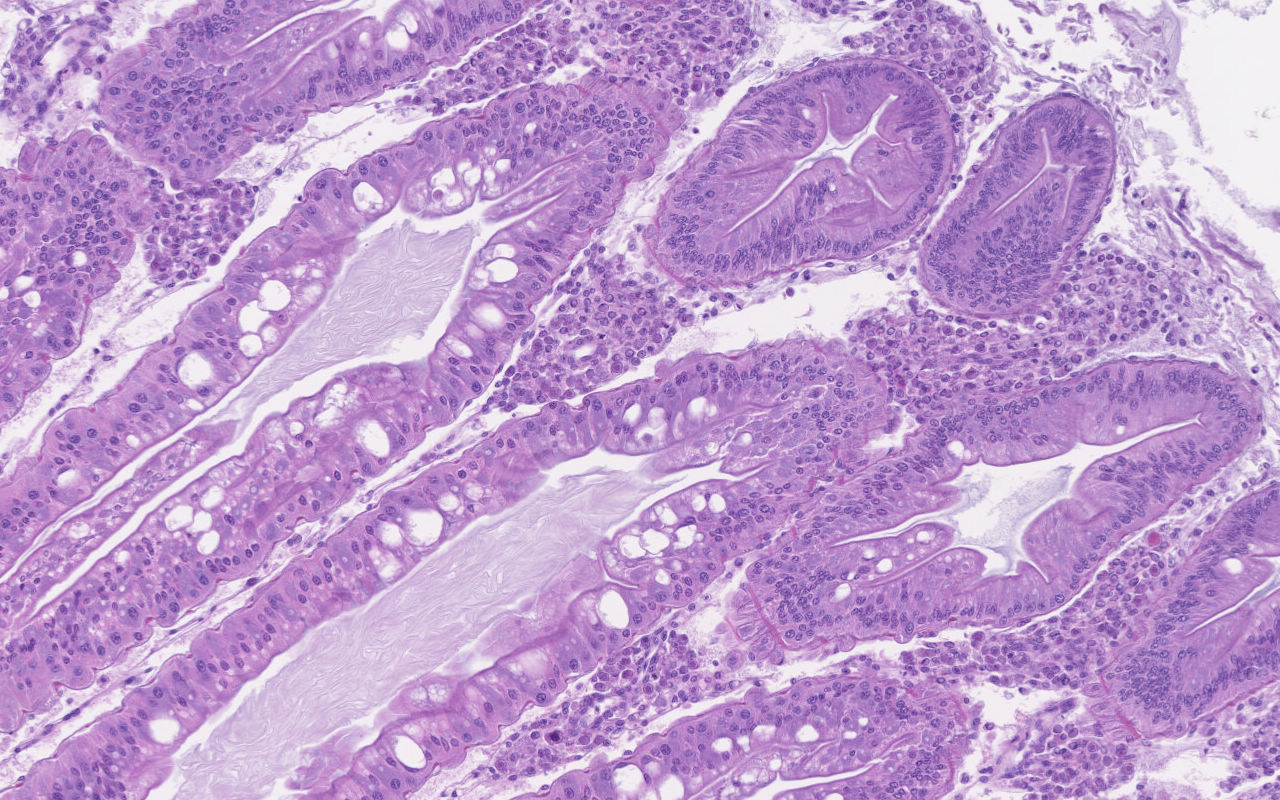
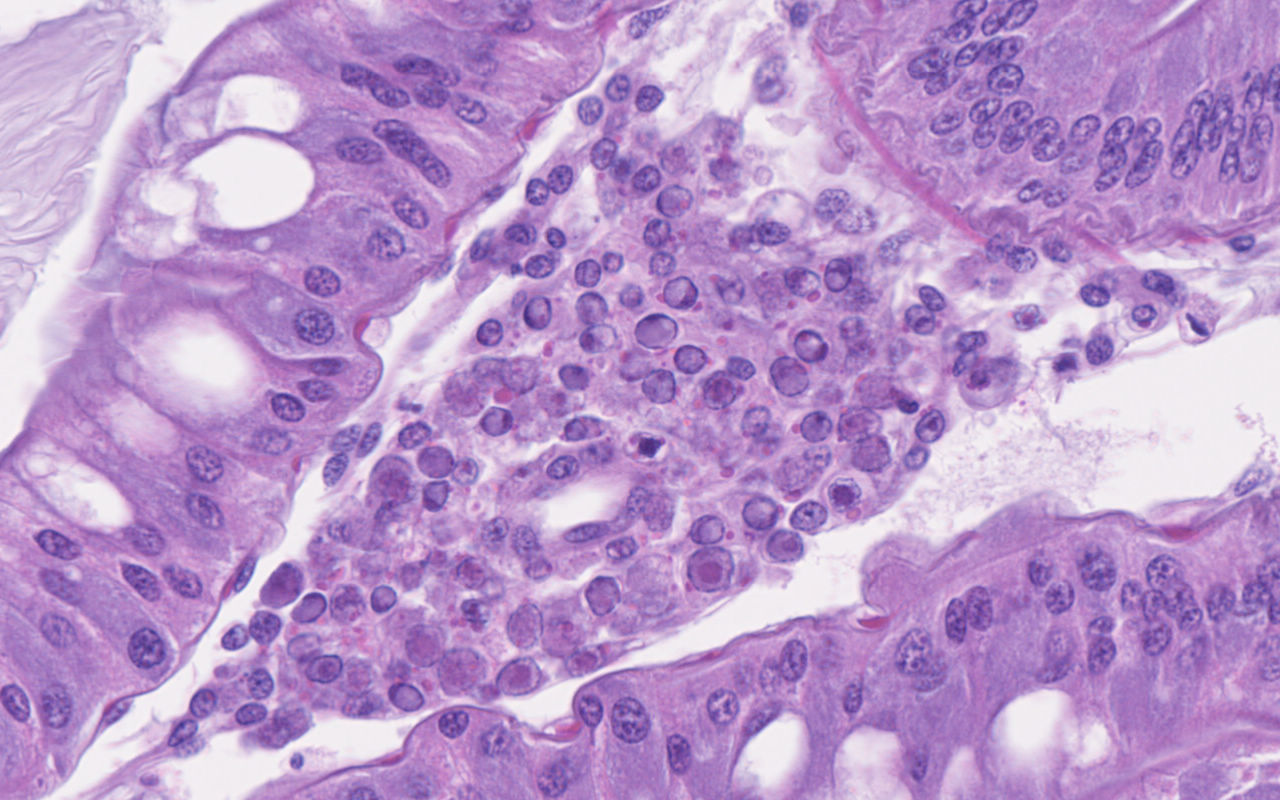
Hepatopancreas: Throughout the hepatopancreas, the hemal sinuses are variably dilated and increased in size in comparison to the adjacent tubules. The dilated sinuses are filled with a moderate to abundant amount of circulating hemocytes and proliferative spongy connective tissues. Fixed phagocytes surrounding the hepatopancreatic tubules are variably enlarged, with multifocal disruption of the typical rosette-like arrangement. Circulating hemocytes, fixed phagocytes, and spongy connective tissue cells often have enlarged, hypertrophic nuclei that contain large eosinophilic to amphophilic inclusion bodies, occasionally surrounded by a clear halo, with margination of the nuclear chromatin along the nuclear membrane (Cowdry-type A inclusion bodies). The cytoplasm of the affected cells often contain smaller, variably-sized, round eosinophilic globules. There are subjectively decreased numbers of reserve inclusion cells throughout the hepatopancreas. In few areas, the epithelial cells lining the hepatopancreatic tubules are variably decreased in size or completely absent, and the lumen contains a moderate amount of basophilic granular material and few individual sloughed epithelial cells.
In addition to the hepatopancreas, similar eosinophilic intranuclear inclusion bodies were observed within spongy connective tissue cells of the exoskeletal membrane (not included in submission).
Contributor Morphologic Diagnosis:
Hepatopancreas: Hepatopancreatitis, moderate, diffuse, chronic, with mild to moderate hepatopancreatic atrophy and numerous eosinophilic intranuclear inclusion bodies
Contributor Comment: As a brief overview, the normal hepatopancreas of most decapod crustaceans is a large compact paired glandular organ that surrounds the midgut and occupies a large portion of the cephalothorax.2 The hepatopancreas is surrounded by a connective tissue membrane and each half consists of two or three lobes comprised of a complex network of blind-ending tubules, which are connected to the midgut gland through common absorptive ducts.2,8 With exception of the distal closed end, each tubule is lined by a single cell layer of epithelial cells, which are divided into subtypes including the embryonic E-cells, fibrillar F-cells, resorptive or absorptive R-cells, and secretory B-cells.2 The embryonic cells reside within the apical portion of the tubule, while the remaining cell types (B-, R-, and F-cells) reside within the digestion zone.8 An outer basement membrane separates the tubules from the hemal sinuses, which contain an arteriole surrounded by a rosette-like structure of fixed phagocytes, which remove foreign material from the hemolymph, and reserve inclusion cells, which contain polysaccharides such as glycogen and proteins such as hemocyanin.3,6,8
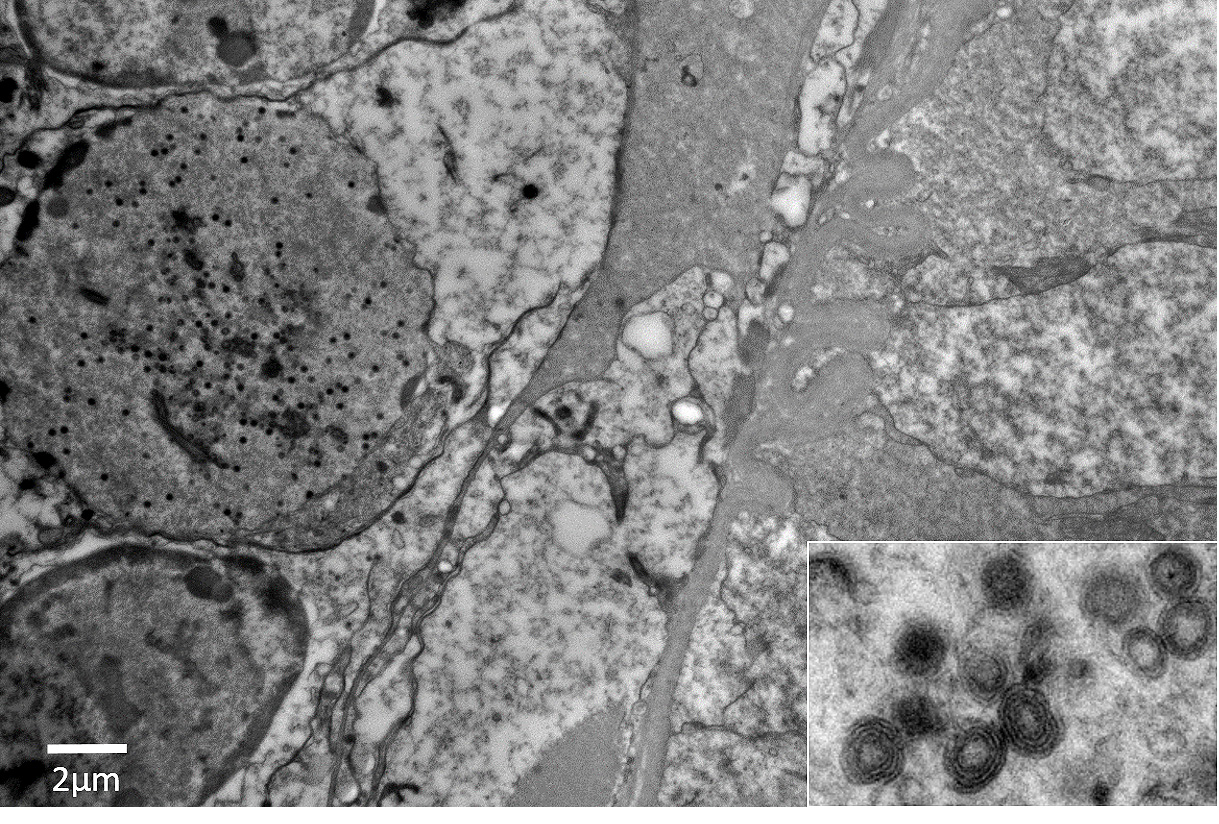
Panulirus argus Virus 1 (PaV1) was discovered approximately 20 years ago as the first naturally occurring pathogenic virus of lobsters.1,3-7 PaV1 was first detected in juvenile Caribbean spiny lobsters (Panulirus argus) from the Florida Keys, Florida, USA, but has since been detected throughout the Caribbean Sea, including reports from St. Croix, St. Kitts, Yucatan (Mexico), Belize, and Cuba.1,3,6 At the time of submission, Caribbean spiny lobsters are the only reported affected host of the virus.6 PaV1 is one of the most significant pathogens affecting spiny lobsters and is believed to be associated with a decline in the spiny lobster fishery in the Florida Keys.6,7 Additionally, these lobsters are shipped internationally, which could play a role in the potential spread of the virus. The virus is not yet classified, but shares morphological characteristics with Herpesviridae and Iridoviridae; it is an unenveloped, icosahedral DNA virus with an approximately 182 nm nucleocapsid that develops within the infected host cellsâ nuclei.6
The virus is most prevalent within and nearly always lethal to the smallest juvenile lobsters, with a decrease in prevalence correlating with increase in the size of lobster; adults may harbor the virus, but do not typically exhibit signs of disease.1,6 Healthy lobsters tend to avoid diseased lobsters, which may explain reduced disease transmission, but may also lead to increased shelter competition and increased predation on the infected lobsters.1,6 Heavily infected lobsters become lethargic and sedentary, cease feeding, and eventually die of metabolic exhaustion.1,6
During early stages of infection, the virus has an apparent predilection for the fixed phagocytes of the hepatopancreas, which later lyse and infect proliferating spongy connective tissue cells and circulating hemocytes, including hyalinocytes and semi-granulocytes.1,3,5 As mentioned above, the fixed phagocytes and circulating hemocytes play an important role in phagocytosing foreign materials from the hemolymph.6,8 The circulating hemocytes affected include hyalinocytes and semigranulocytes; granulocytes, as well as fibrous connective tissue cells are not affected.1,3 All affected cell types are derived from the mesoderm, suggesting that the virus may have a tropism for this developmental germ layer.6 In heavier infections, virtually all spongy connective tissue cells are infected and hepatopancreatic tubules become atrophied, with depletion of reserve inclusion cells, leading to metabolic wasting and death.6
The hemolymph of affected lobsters has a characteristic white, milky appearance and fails to clot.6,7 Histopathologic lesions can be observed within 10-15 days of infection and include alterations to the fixed phagocytes and spongy connective tissue with enlarged nuclei containing Cowdry-like inclusion bodies.8 Molecular diagnostics including polymerase chain reaction (PCR) and fluorescent in situ hybridization (FISH) have been utilized for disease confirmation.4-6
In this case, the abundance of intranuclear eosinophilic inclusion bodies coupled with the positive PCR result were diagnostic for Panulirus argus Virus 1 (PaV1). In addition to the intranuclear inclusions, eosinophilic globular material was observed within the cytoplasm of the infected cells. The origin of this material has yet-to-be determined, but based on electron microscopy, we believe this material could be virus-induced particles within the cytoplasm. As another alternative, the histologic appearance is somewhat similar to the eosinophilic globular aggregates associated with reserve inclusion cells and therefore, these globules may represent polysaccharide aggregates such as glycogen. The areas with loss of hepatopancreatic epithelium and intraluminal basophilic granular material are believed to be euthanasia-induced artifacts, though virally-induced change cannot be ruled out. However, PaV1 has not been reported to affect hepatopancreatic epithelium, so this is considered less likely.
Contributing Institution:
Louisiana Animal Disease Diagnostic Laboratory (LADDL), School of Veterinary Medicine, Louisiana State University (http://www1.vetmed.lsu.edu/laddl/index.html)
JPC Diagnosis: 1. Hepatopancreas:
Hepatopancreatitis, interstitial, hemocytic, diffuse, moderate, with
numerous eosinophilic intranuclear inclusion bodies.
2. Hepatopancreas, gill, midgut; circulating hemocytes: Eosinophilic intranuclear inclusion bodies.
3. Hepatopancreas: Atrophy, tubular, diffuse, severe.
JPC Comment: The contributor has done an outstanding job of describing the pertinent anatomy of the decapod hepatopancreas as well as illustrating this important disease of spiny lobsters. Many pathologists are likely unfamiliar with the anatomy of the lobster, and are directed to reference 8 below, an excellent review of the gross and histologic anatomy of the lobster at: https://scholarworks.wm.edu/cgi/viewcontent.cgi?article=1005&context=reports (reference 8)
Many
pathologists (as well as the majority of confrence participants) are largely
unaware of the diseases of spiny lobsters. In addition to Panulirus argulus
virus -1, spinylobsters are host to a number of infectious, parasitic, and
syndromal conditions of interest.
At
this time, PaV1, appears to be the only natural viral infection of spiny
lobsters, although it appears they may be natural hosts for white spot syndrome
virus, a viral disease of shrimp.6
Spiny lobsters are host however, to a number of bacterial pathogens. Aerococcus
viridans is a gram-positive bacillus that is most common in holding
facilities but has been identified in Cuban spiny lobsters. This agent results
in a reddish disocloration of the shell, pink hemolymph and coagulopathies when
introduced through a broken exoskeleton. âShell diseaseâ is a condition caused
by a numer of chitinoclistic gram-negative bacterial, including bacteria from
the genera Vibrio, Aeromonas, Pseudomonas, and Shewanella. This
multifoal erosion of the shell may occur randomly on the exoskeleton, or in
spiny lobsters, affect the tail fan. In addition, Vibrio sp. may cause
disseminated disease in spiny lobsters, especially larvae, but also may be
cultured from hemolymph in apparently normal individuals of this species. Fouling
bacteria, such as Leucothrix mucor damage eggs and larvae of lobsters in
culture. Several species of microsporidia are also seen in the spiny labsters,
and may affect the meat quality of infected animals.6
A number of fungi infect spiny lobsters,. Oomycetes and phycomycetes usually
infect the eggs and larvae, and are more common when water quality is poor. Fusarium
solani results in black shell disease, melanization which is often seen in
a variety of diseases affecting the exoskeleton in this species. Cilites are
often found as commensals on the exterior of lobsters particular in cultures.6
A number of helminth parasites are described in spiny lobsers, to include digenetic trematodes, which utilitze them as intermediate hosts and encyst as metacercariae in the msuculature. Connective tissues of metacestodes may encyst in connective tissues. Rotifers, copepods, and amphipods are often symbionts or commensals in spiny lobsters or their egg clutches.6
Disease syndromes in lobsters generally are associated with interesting names, even if their etiology is unknown. âTurgid lobster syndromeâ occurs in spiny lobsters in New Zealand and Australia, is characterized by swelling of the arthrodial membranes, and a causative agent is unknown. Australia is also host to âpink lobster syndromeâ in which the meat turns pink to yellow and is unpalatable. Mass mortality evens have been documented by habitat alteration such as hypoxia events, algal blooms, and mass strandings.6
The moderator reviewed the anatomy and histology of the lobster. In discussing the most appropriate morphologic diagnosis for this case, the moderator discussed a preference for the term âhemocyticâ rather than granulocytic due to the difficulties in distinguishing the two on routine light microscopy. Atrophy of the hepatopancreas in this case is especially profound as normal hepatopancreas is filled with lipid vacuoles, and few remained in this particular specimen, but it was added as a separate morphologic diagnosis as it was difficult to establish whther it resulted from the viral infection, or simply diminished nutritional status as a consequence of captivity.
References:
1. Behringer DC, Butler MJt, Shields JD, Moss J. Review of Panulirus argus virus 1--a decade after its discovery. Dis Aquat Organ. 2011;94: 153-160.
2. Gibson R, Barker PL. The Decapod Hepatopancreas. Oceanogr Mar Biol Ann Rev. 1979;17: 285-346.
3. Li C, Shields JD, Ratzlaff RE, Butler MJ. Pathology and hematology of the Caribbean spiny lobster experimentally infected with Panulirus argus virus 1 (PaV1). Virus Res. 2008;132: 104-113.
4. Li C, Shields JD, Small HJ, et al. Detection of Panulirus argus Virus 1 (PaVI) in the Caribbean spiny lobster using fluorescence in situ hybridization (FISH). Dis Aquat Organ. 2006;72: 185-192.
5. Montgomery-Fullerton MM, Cooper RA, Kauffman KM, Shields JD, Ratzlaff RE. Detection of Panulirus argus Virus 1 in Caribbean spiny lobsters. Dis Aquat Organ. 2007;76: 1-6.
6. Shields JD. Diseases of spiny lobsters: a review. J Invertebr Pathol. 2011;106: 79-91.
7. Shields JD, Behringer DC, Jr. A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Dis Aquat Organ. 2004;59: 109-118.
8. Shields JD, Boyd RA: Atlas of Lobster Anatomy and Histology. In: Special Papers in Marine Science. Virginia Institute of Marine Science, College of William and Mary, 2014