Signalment:
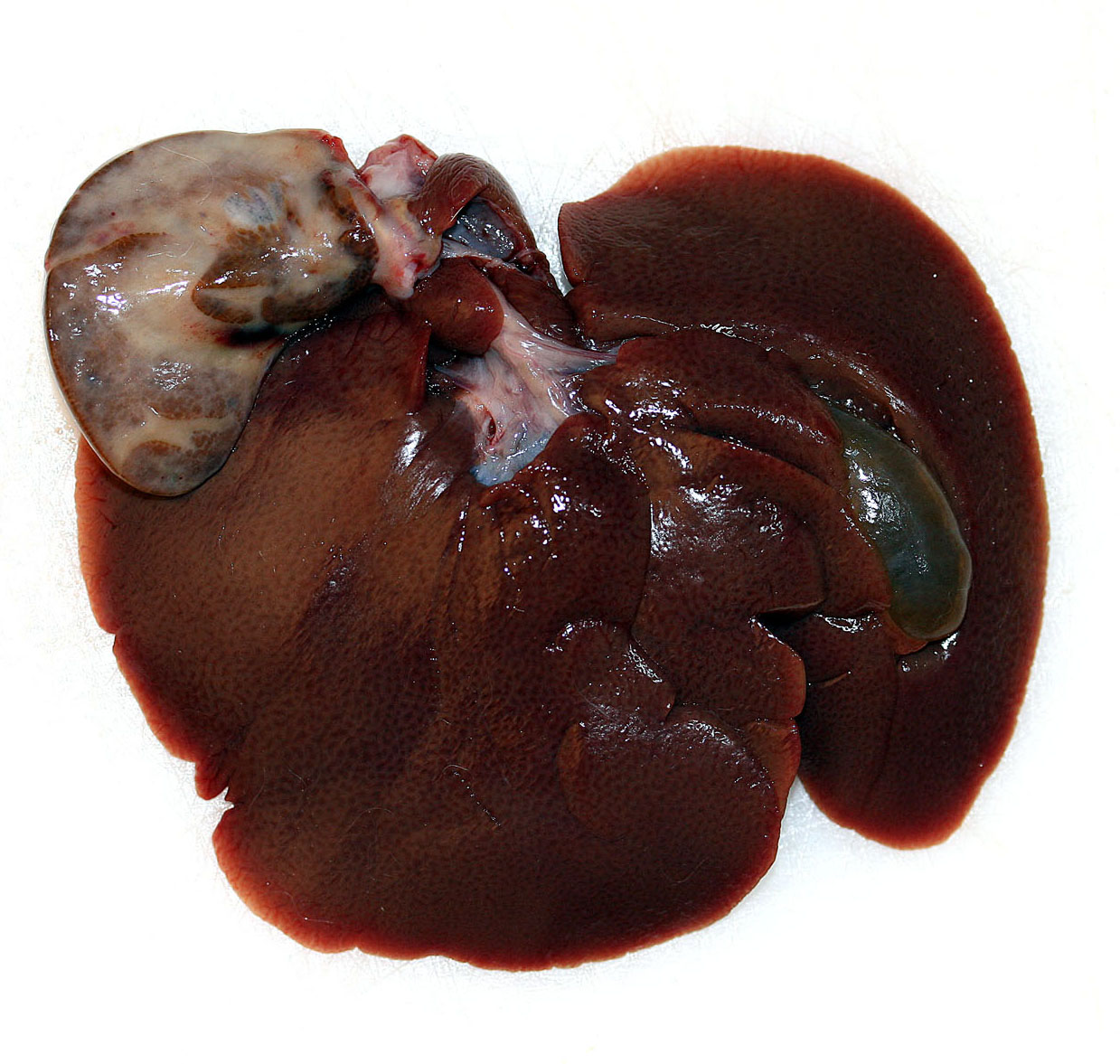
Gross Description:
Histopathologic Description:
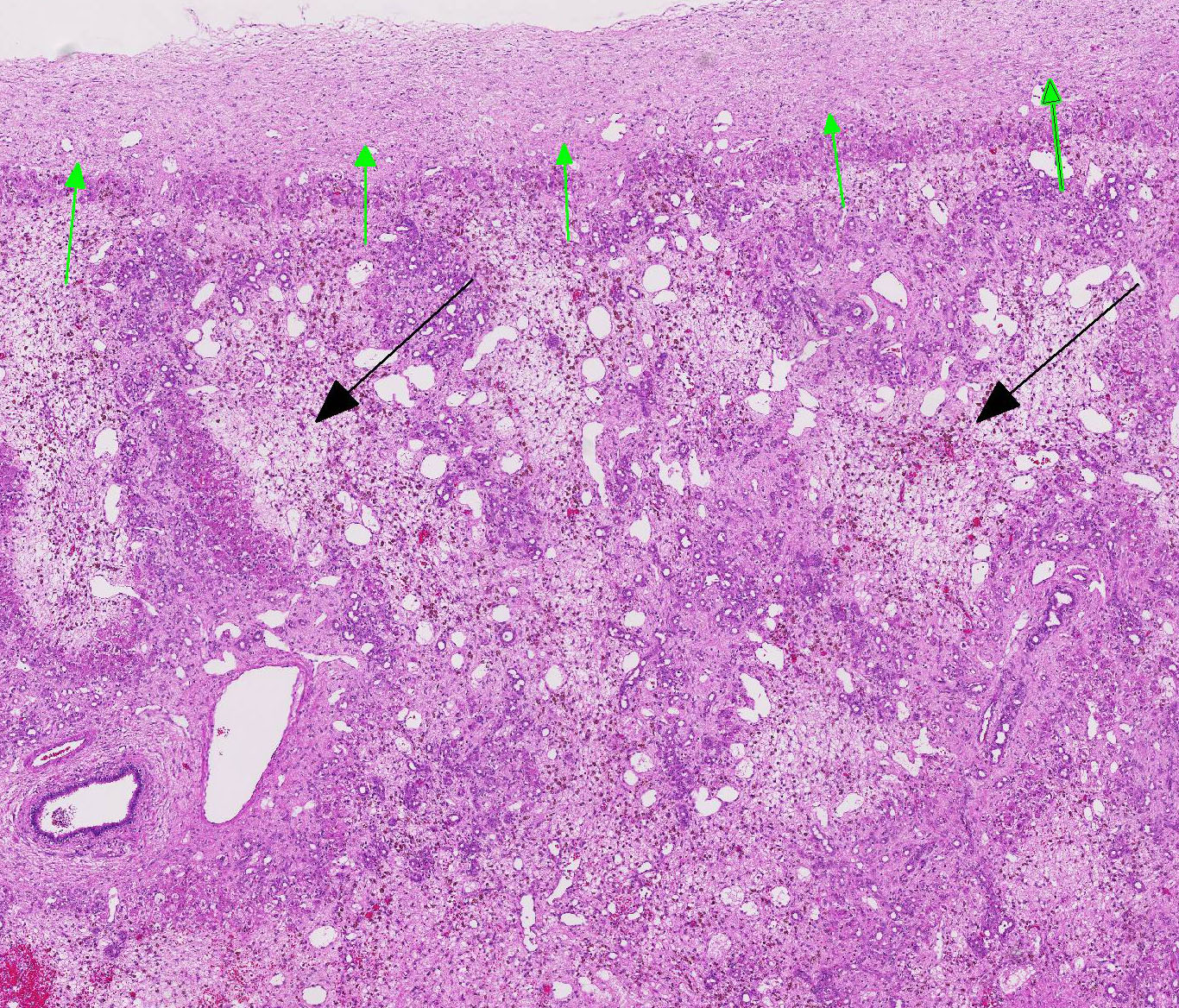
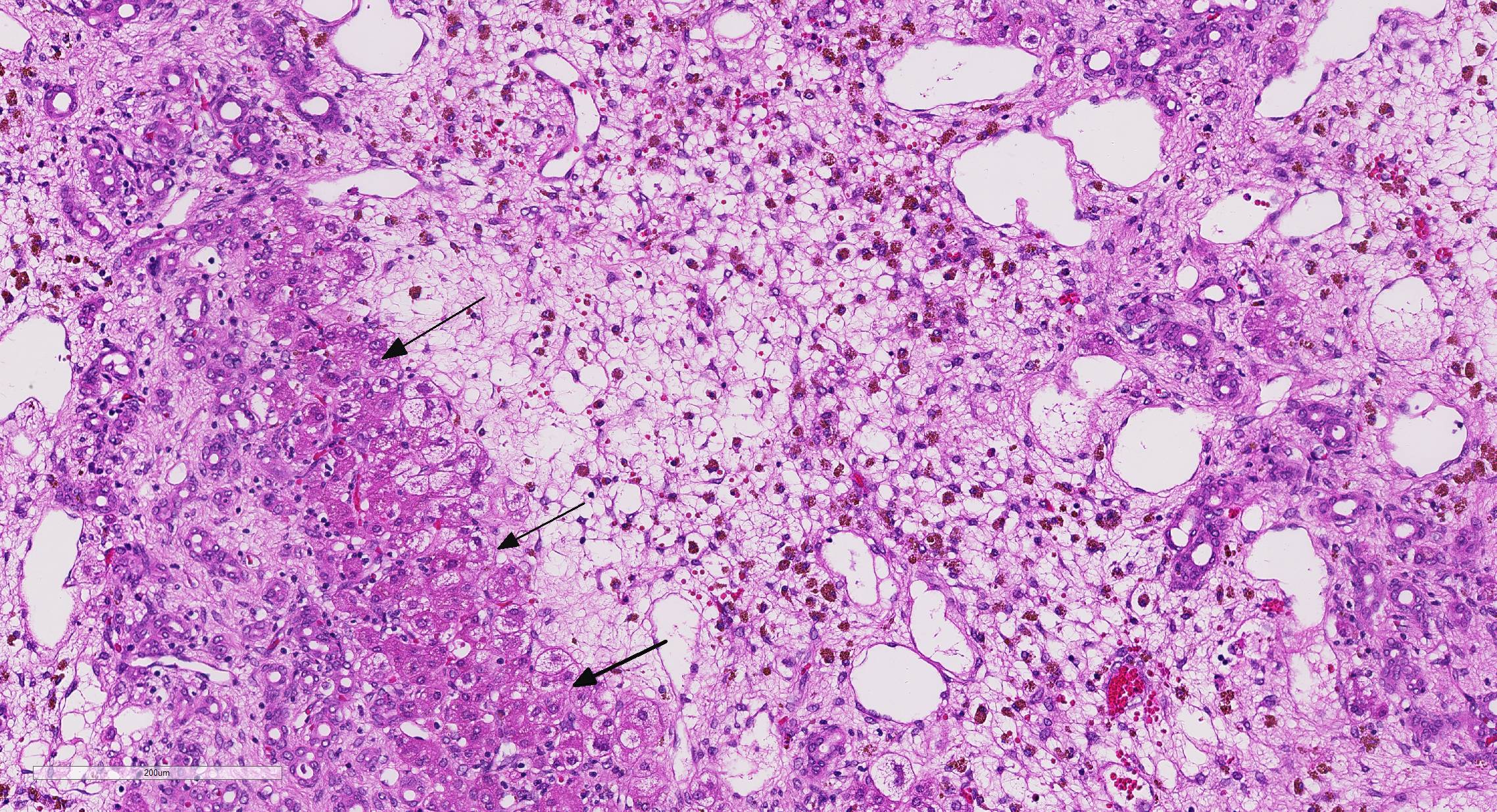
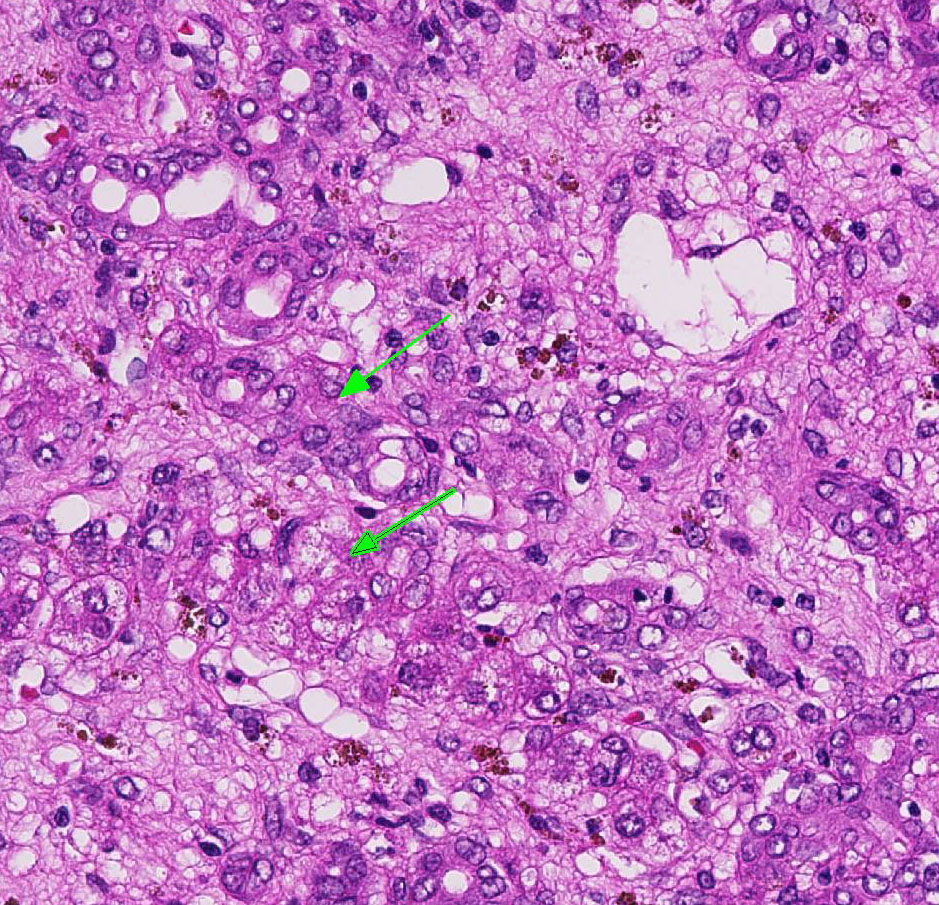
There is mild, multifocal, centrilobular sinusoidal dilatation by erythrocytes (con-gestive hyperemia), as well as mild multifocal accumulations of extravascular erythrocytes (hemorrhages) and a moderate, diffuse infiltration by macrophages, mostly stuffed with multiple, cytoplasmic, brown granules (hemosiderosis), and lesser and variable concentrations and combinations of lymphocytes, plasma cells, and heterophils.
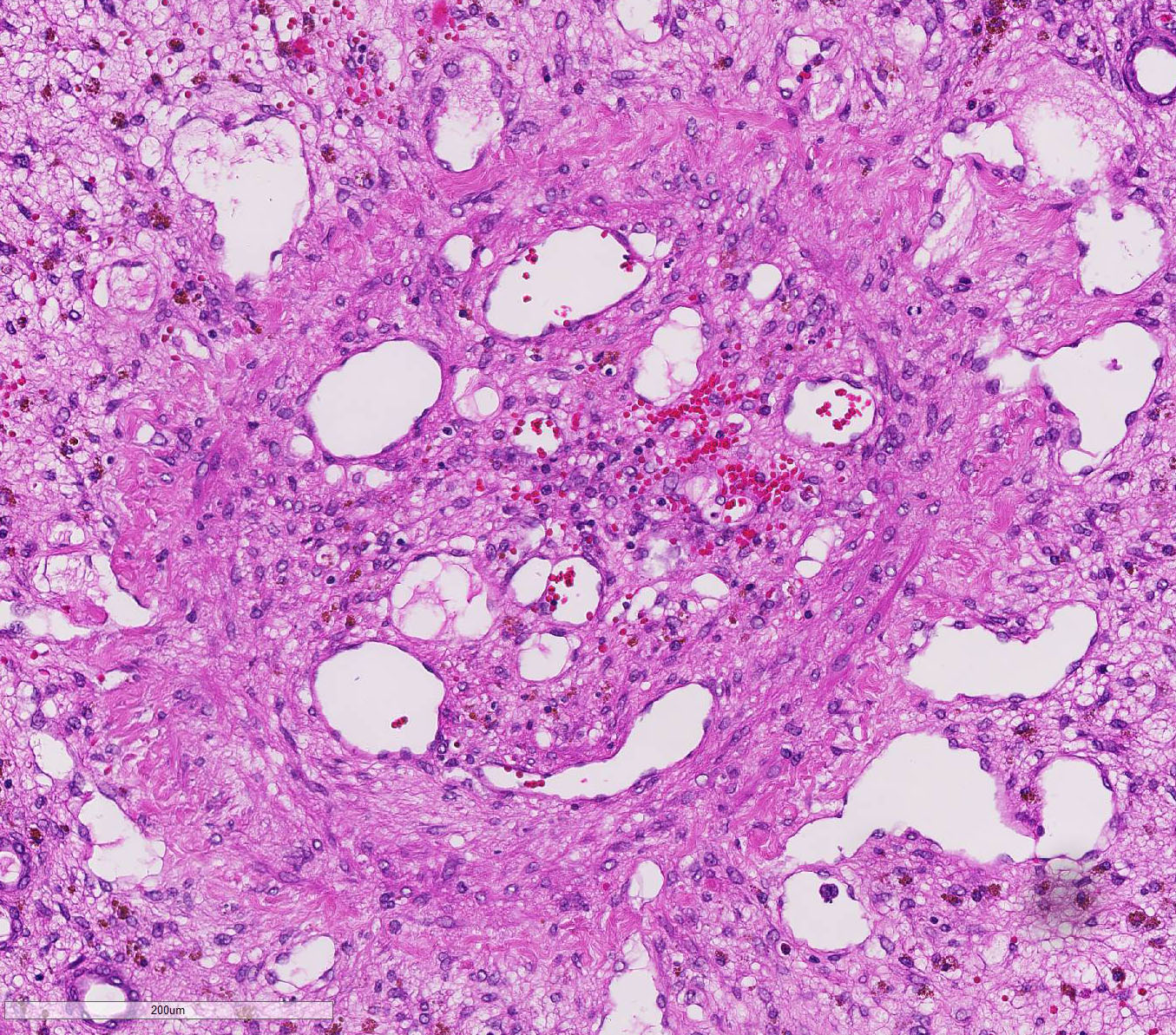
There is a moderately increased amount of periportal bridging bundles of collagen fiber-rich tissue (fibrosis), a severely in-creased number of portal bile ducts (biliary hyperplasia), moderately dilated lymphatic vessels, and severe thickening of the hepatic capsule (fibrosis).
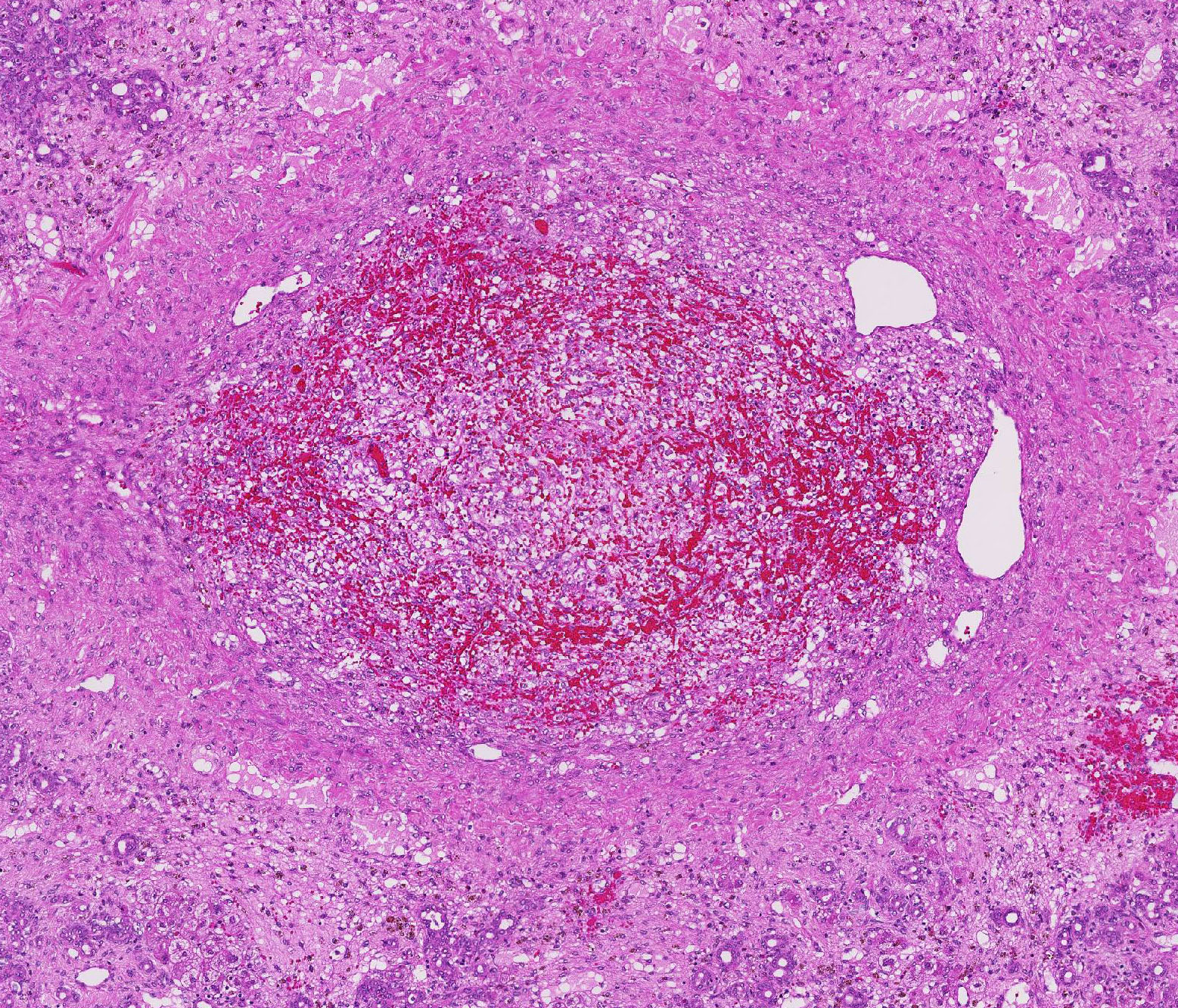
There is a centrally located, 500 µm in diameter, relatively thin-walled blood vessel (vein) which is completely occluded by a variable amount of a densely packed, hyper-eosinophilic, fibrillary to beaded mass (fibrin-rich thrombus), which is partially and/or completely (depending on the slide) replaced by fibroblasts and numerous small caliber blood vessels with hypertrophic endothelium (granulation tissue), mildly infiltrated by hemosiderin laden macro-phages (hemosiderosis).
The other section of liver is unremarkable.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Epidemiology: Liver lobe torsion occurs rarely in many species including rabbits, dogs, cats, pigs, horses, otters, rats, mice and human beings.4 Clinical course: Clinical sequelae of liver lobe torsion range from subclinical, to an acute crisis with lethargy, abdominal pain, jaundice, anorexia, vomiting, collapse and sudden death.7,8 Immediate partial hepatectomy is suggested to be the treatment of choice in symptomatic rabbits.6 Pathogenesis: Liver lobe torsion results in constriction of venous return leading to venous and/or arterial thrombosis, marked venous congestion, infarction, and ischemic necrosis of the affected lobe.2 Affected rabbits may develop shock, abdominal hemorrhage, and/or septic peritonitis leading to acute cardiovascular failure and death. Liver lobe torsion may be diagnosed incidentally in surviving animals. These animals will have lobular hepatocellular atrophy, fibrosis, and chronic inflammation.8
JPC Diagnosis:
Conference Comment:
When hepatic injury is limited and the reticulin meshwork is intact, regeneration of mature hepatocytes is stimulated by hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor-α (TGF-α), and insulin-like growth factor (IGF).2 Interleukin-6 (IL-6) secreted by TGF-α activated Kupffer cells also stimulates hepatocellular regeneration.2 Once hepatic mass has been replaced, transforming growth factor-β (TGF-β) inhibits ongoing proliferation of hepatocytes. In this case, there are occasional periportal multi-nucleated and vacuolated hepatocytes, suggesting attempted regeneration and deg-eneration.2
Ductular reaction is a similarly complex process. In severe liver injury with ex-tensive loss of mature hepatocytes, there is stimulation of bipotential progenitor cells (oval cells in rodents), which normally reside in the periportal canals of Hering.2,3,5 The canals conduct bile from bile canaliculi to bile ducts and are partially lined by hepatocytes and biliary epithelium.2,5 Diff-erentiation of bipotential progenitor cells, as well as de-differentiation and biliary metaplasia of mature hepatocytes, in response to severe liver damage is the basis for biliary ductular reaction patterns.2,5
Conference participants briefly disussed the types of ductular reactions and a relatively new classification pattern proposed by Desmet.3 Ductular reactions are classified into type 1, in which mature bile ducts proliferate secondary to cholestasis; type 2 which is hepatocyte ductular metaplasia induced by in-flammation, intoxication, or hypoxia; and type 3, which is the activation and proliferation of liver bipotential progenitor cells in response to massive hepatic parenchymal loss.2,3 In this case, the ductular reaction likely started as type 2 and likely progressed to type 3 in response to chronic venous occlusion, hypoxia, fibrosis, and diffuse severe hepatocellular loss sec-ondary to hepatic torsion.2,3,5
Finally, hepatic fibrosis also begins at the molecular level. Hypoxic hepatocytes induce hypoxia-inducible factor 1-α (HIF-1 α) and platelet derived growth factor (PDGF) to activate hepatic stellate cells within the space of Disse.2 These cells then transform into myofibroblasts resulting in progressive diffuse hepatic fibrosis.2 This stimulates centrilobular and periportal hepatocytes to dedifferentiate and eventually assume a biliary ductular phenotype.3 On-going hypoxia and fibrosis then results in massive parenchymal loss. Remaining bi-potential progenitor cells in the canals of Hering then differentiate toward the biliary duct phenotype rather than differentiating to mature hepatocytes.3 Persistence of ductular reaction further contributes to portal and periportal fibrosis through secretion of previously mentioned profibrogenic growth factors.2
References:
2. Cullen JM, Stalker MJ. Liver and Biliary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 258-353.
3. Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch 2011; 458:251-259.
4. Graham J, Basseches J. Liver Lobe Torsion in Pet Rabbits. Clinical Consequences, Diagnosis and Treatment. Vet Clin Exot Anim. 2014; 17:195-202.
5. Rothuizen J, Bunch SE, Charles JA, et al. Morphological classification of parenchymal disorders of the canine and feline liver: 1. Normal histology, cirrhosis. In: WSAVA Standards for clinical and histological diagnosis of canine and feline liver disease. St. Louis, MO: Elsevier; 2006:78-79, 85-88.
6. Stanke NJ, Graham JE, Orcutt CJ, Reese CJ, Bretz BK, Ewing PJ, Basseches J. Successful outcome of hepatectomy as treatment for liver lobe torsion in four domestic rabbits. JAVMA. 2011; 238:1176-1183.
7. Wenger S, Barrett EL, Pearson GR, Sayers I, Blaey C, Redrobe S. Liver lobe torsion in three adult rabbits. J Small Animal Practice. 2009; 50:301- 305.
8. Weisbroth SH. Torsion of the Caudate Lobe of the Liver in the Domestic Rabbit (Oryctolagus). Vet Pathol. 1975; 12:13-15.