WSC 22-23
Conference 20
Case III:
Signalment:
Male, Sprague-Dawley rat (Rattus norvegicus)
History:
This rat was administered a test article to induce seizures in order to evaluate the effectiveness of anticonvulsant therapeutics.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
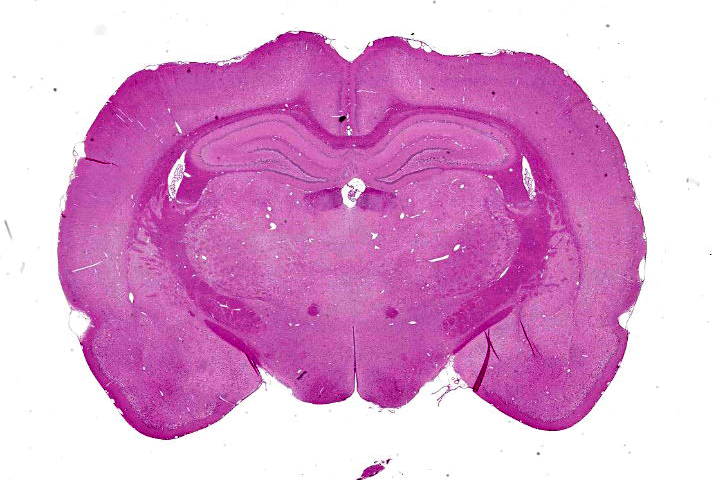
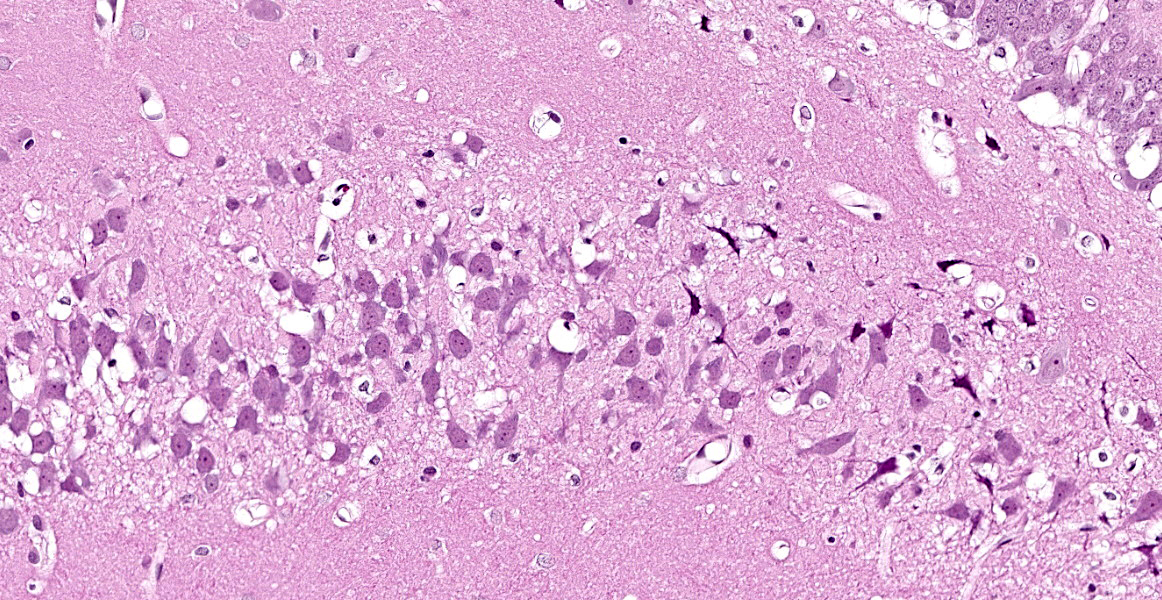
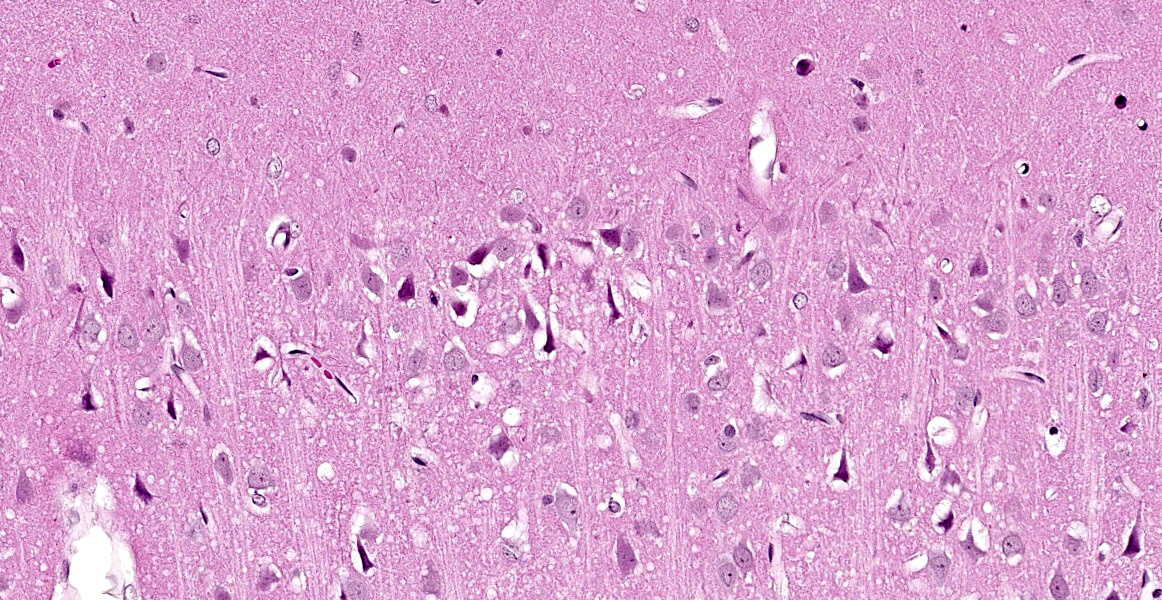
Cerebrum at the level of the thalamus and hippocampus: Multifocally within the cerebral cortex, piriform cortex, amygdala, hippocampus, thalamus, caudate nucleus, and putamen, there is neuronal degeneration and necrosis characterized by shrunken, angular, hypereosinophilic cytoplasm of neuron cell bodies. There is rarefaction of the white matter immediately adjacent to affected neuron cell bodies.
Contributor’s Morphologic Diagnoses:
Cerebrum at the level of the thalamus and hippocampus: Neuronal degeneration and necrosis, multifocal, severe, with rarefaction.
Contributor’s Comment:
Due to low cost and ease of manufacturing of chemical warfare agents, there is an increased likelihood of use of chemical warfare agents by terrorists, as well as, by various governments. Relatively recent examples of chemical attacks include the use of chlorine gas by Al Qaeda in 2006 and 2007, the use of various chemicals by ISIS in 2015 and 2016, and the poisoning of a former Russian agent in 2018. One of the most notable examples of a chemical warfare agent being deployed is the use of the nerve agent sarin by members of the Aum Shinrikyo religious cult in Japan in 1995 that killed 12 and sickened 5,000 others. In order to decrease the likelihood of future chemical warfare agent use there is a focus on strategies to research and mitigate the negative physiologic effects of various agents so that their deployment is useless as effective antidotes are readily available.
Mechanism of Action of Nerve Agents. The chemical warfare (CW) nerve agents include the anticholinesterase nerve agents tabun (GA), sarin (GB), soman (GD), cyclosarin (GF), and VX, all of which are, or have been, part of the US domestic munitions inventories.8 These agents are potent anticholinesterase compounds deliberately formulated to induce debilitating effects or death during wartime hostilities and have been used by military authorities of several nations to develop munitions (e.g., Germany during the Nazi era, the USA, and the former Soviet Union).8
All of the listed nerve agents are anticholinesterase compounds and induce accumulation of the neurotransmitter acetylcholine (ACh) at neural synapses and neuromuscular junctions by of nerve agents can result in excessive bronchial, salivary, ocular and intestinal secretions, sweating, miosis, bronchospasm, intestinal hypermotility, bradycardia, muscle fasciculations, twitching, weakness, paralysis, loss of consciousness, convulsions, depression of the central respiratory drive, and death.8
Nerve Agent-Associated Injury to the Nervous System. Nerve agents exert their neuropathologic effects through binding and irreversible inactivation of acetylcholinesterase (AChE), the enzyme that hydrolyzes ACh, leading to a toxic accumulation of ACh at nicotinic (skeletal muscle and preganglionic autonomic) receptors, muscarinic (mainly postganglionic parasympathetic) receptors, and central nervous system synapses.9
Historically, this has been of concern in organophosphate and carbamate insecticide exposure among farm workers; however, it is likely that use of nerve agents in civilian populations or against military personnel would result in a range of exposures that stem from both the proximity of various groups to the site of deployment and the persistence of residues of some agents in the environment.9
Common nerve agents developed for chemical warfare purposes include the G-series (so names because they were first developed by German scientists in the mid-1930s) and V-series (a designation of more ambiguous origin) weapons.9 As previously mentioned, the G-series includes tabun (GA), sarin (GB), soman (GD), and cyclosarin (GF), while the V-series includes VX, first synthesized by the British in 1954.9 The G-series compounds are volatile liquids at room temperature, are soluble in both fat and water, and are absorbed readily through the eyes, respiratory tract, and skin.9 V-series agents are viscous and toxic mainly via skin exposure, and therefore pose a lower inhalation hazard than the G-agents; however, they are notoriously persistent in the environment.9
Acute exposure to nerve agents is associated with a range of clinical symptoms, varying from abnormal movements and salivation to limb tremor and muscle fasciculations, to convulsions.9 In a variety of animal and especially rodent models, animals that survived seizures tended to manifest extensive bilateral brain neuronal necrosis that affected predominantly the forebrain, thalamus, tegmentum, and spinal cord.9 Acute injury may be attributable to several mechanisms.9
Ischemic hypoxia may derive from respiratory insufficiency during prolonged seizures, and evidence of cellular ischemia is present in brains of exposed animals.9 This consists of shrinkage of the cell soma and proximal dendrites, cytoplasmic microvacuolation due to mitochondrial swelling, dispersion of Nissl substance (cytoplasmic RNA), increased cytoplasmic eosinophilia, nuclear changes including displacement of the nucleus to an eccentric position in the neuron, shrinkage, and darkening.9 Generally, these nerve agent-associated lesions were described as being indistinguishable from those associated with brain ischemia or anoxia.9
Within minutes after exposure to nerve agents, there is a marked decrease in AChE activity and associated rise in ACh.9 The earliest seizure activity begins in the absence of other significant neurotransmitter alterations and is prevented by anticholinergic drugs.9 These observations suggest that seizure-associated neuropathologic findings that occur upon nerve agent exposure are caused primarily by a mechanism of cholinergic toxicity.9
However, if seizures progress untreated, other neurotransmitter systems display secondary alterations, and the involvement of these has been invoked in models of injury to cerebrum that involve mechanism of “excitotoxic” injury.9 Specifically, these refer to the involvement of the excitatory amino acid transmitter glutamate, which increases intracellular calcium mobilization.9
In excitotoxicity, overstimulation of glutamatergic synapses leads to marked neuronal calcium dyshomoeostasis that in turn leads to neuronal injury.9 Importantly, pretreatment or early post-exposure treatment of experimental animals with anticonvulsants (e.g., benzodiazepines such as diazepam), blocks nerve agent-associated seizures which in turn prevents or diminishes neuropathologic effects.9 This occurs in the absence of a direct effect on cholinergic processes.9 These observations, taken together, suggest strongly that excitotoxic mechanisms contribute largely to the structural changes observed in the cerebrum upon nerve agent exposures.9
Abrogation of these mechanisms by anticholinergic drugs within 20-40 min of the onset of seizures is sufficient in most cases to significantly diminish neuropathologic lesions.9 The failure of anticholinergic drugs to prevent nerve agent-associate pathologic changes after this time period has been attributed to the recruitment and dominance of non-cholinergic mechanisms of excitotoxicity and perhaps to secondary loss of the integrity of the blood-brain barrier.9
Technical Considerations for Toxicologic Neuropathology. Cost-effective, high-quality neuropathologic evaluations require teamwork involving the recording and assessment of detailed clinical signs and gross postmortem observations, careful and timely collection and processing of tissues, and a working knowledge of both neuroanatomy and neuropathology.4 The process begins well before the necropsy.4 It is important for the pathologist to have knowledge of the likely target effects (distribution, biochemistry, secondary changes) of the compound so that tissue collections and evaluations can be optimized.4
Evaluation of murine brain tissue requires considerable expertise in the preparation of tissue samples prior to examination by a neuropathologist. The use of whole-animal perfusion techniques and specialized grossing implements (such as brain matrices) are practical and effective tools which can help ensure that virtually identical brain regions are collected and prepared from each animal within a large study.
As with many other organ systems, the most common way for nervous tissue to be fixed is by direct immersion in an aldehyde fixative solution.3 However, whole body perfusion via the vascular system is preferred, particularly if any degree of special detail is necessary in the study.3 Once mastered, perfusion yields reproducible, high-quality preparations of nervous tissue.3 However, this approach requires technical dedication and practice before mastery.3 The technique cannot simply be written and given to an inexperienced technical specialist for execution without proper instruction and training.3 An excellent summary of the practical aspects of whole body perfusion is provided in the below referenced article by Fix and Garman.3
Investigators seeking to evaluate brain injuries will select a region of interest using a standard brain atlas which localizes the desired coronal section by its relationship to the sutures of the skull. By definition, the Bregma level refers to the junction of the coronal and sagittal sutures on the skull, and a coronal brain section at this level corresponds to a Bregma level of 0.0 mm.4 Bregma coordinates represent the number of millimeters rostral (positive numbers) or caudal (negative numbers) to this plane.4
Rat brain atlases are developed using rats of a particular sex, strain, and weight class (e.g., adult, male, medium-sized [270 to 310 g], Wistar rats were used for Paxinos and Watson’s The Rat Brain in Stereotaxic Coordinates, 5th edition).6 For this reason, if rats of a different sex, strain, or weight class are used, stereotaxic accuracy will be affected.6 In other words, if the rat brain is smaller than the medium-sized rat used for the reference atlas, the distance from the Bregma to the desired coronal section will need to be proportionately scaled down to acquire the region of interest.
Even with a good brain atlas, the process of actually matching slides on the microscope with sites depicted in the atlas can be challenging.4 Since histologic sections rarely match the exact plane of section depicted in an atlas, the pathologist must have a basic working knowledge of neuroanatomy and the ability to envision how the landmarks will be shifted when sections are tilted or skewed from the true coronal planes typically presented in the atlases.4 A series of atlases coauthored by Paxinos and Watson provides good neuroanatomic references for rodents, and is available in both hard copy and in electronic format.4
Once the target tissue within the brain has been fixed, grossed, and embedded, the technical skill in the histology laboratory is perhaps the most important factor in arriving at a mounted section of the specific region of interest. The target tissue must be embedded with sufficient excess tissue to allow for block facing and sectioning through the block until the desired serial section is mounted on the glass slide.
The skill level of the histology technician cannot be overstated in murine brain research. First, the individual must have a detailed understanding of murine neuroanatomy. Second, he or she must possess the academic interest to study reference brain atlases and understand where the region of interest is located relative to other structures. Third, the technician must be able to recognize detailed brain structures in unstained paraffin?embedded tissue sections. Finally, the technician must be able to make adjustments if the animal’s size or gender do not match the reference atlas. Microtomy is, by its nature, a destructive process since the technician must section through the block until the desired section is exposed. Because of this, the technician must know when to stop sectioning so that the region of interest is not inadvertently consumed.
For pathologists with an interest in toxicologic neuropathology, there is an excellent compilation of resources, designed to consolidate a broad range of useful neurobiology, neuropathology, and neurotoxicology resources in a single reference.1
Contributing Institution:
https://usamricd.apgea.army.mil/
JPC Diagnosis:
Cerebrum, hippocampus and thalamus: Neuronal degeneration and necrosis, multifocal and segmental, with rarefaction.
JPC Comment:
The moderator remarked on the remarkable preservation of this tissue achieved by perfusion and described the histologic evidence of perfusion: dilated (non-retracted) empty blood vessels and reduced dark neuron artifact.
This case illustrates the classic but nonspecific lesion of acute eosinophilic (acidophilic) neuronal degeneration and necrosis. In this case, neuronal necrosis is due to the effects of a nerve agent, which, as the contributor describes, works in a few ways: by causing direct neuronal acetylcholine toxicity by inhibiting acetylcholinesterase, by causing excitatory nerve injury, and by causing ischemia due to respiratory depression secondary to prolonged seizure activity. Acute eosinophilic neuronal necrosis can occur in other conditions as well, including ischemia or hypoxia, thiamine deficiency, heavy metal toxicosis (including lead and organic mercury), hypoglycemia, and inflammation.5,7 Neurons are acutely sensitive to ischemic and hypoxic injury because they maintain minimal energy stores.2 When deprived of oxygen due (i.e. ischemia or cardiac arrest), the most sensitive neurons in the cerebral cortex die within 10 minutes due to decreased ATP generation.2 Neurons in the cerebral cortex, Purkinje cells, and the hippocampus are the most sensitive to ischemic injury; this phenomenon is referred to as selective neuronal vulnerability.2,5 Other causes of decreased ATP generation leading to neuronal necrosis include cyanide, which interferes with mitochondrial cytochrome oxidase activity during cellular respiration, and carbon monoxide, which prevents oxygenation of peripheral tissues. 2,5 For the same reason, hypoglycemia (i.e. due to a functional insulinoma) also causes neuronal necrosis, and, in general, neurons display the same sensitivity to hypoglycemia as ischemia (with the exception of Purkinje cells, which are more resistant to hypoglycemic injury).2
References:
- Bolon B, Bradley A, Garman RH, Krinke GJ. (2011). Useful toxicologic neuropathology references for pathologists and toxicologists. Toxicol Pathol 2011;39(1): 234-239.
- Cantile C, Youssef S. Nervous system. In: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:253-254.
- Fix AS, Garman RH. Practical aspects of neuropathology: a technical guide for working with the nervous system. Toxicol Pathol. 2000;28(1):122-131.
- Jordan WH, Young JK, Hyten MJ, Hall DG. Preparation and analysis of the central nervous system. Toxicol Pathol. 2011;39(1):58-65.
- Miller AD, Porter BF. Nervous System. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 7th ed. St. Louis, MO: Elsevier Mosby; 2022: 904-905,934.
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 5th ed. San Diego, CA: Elsevier Academic Press; 2005.
- Vandevelde M, Higgins RJ, Oevermann A. Veterinary Neuropathology: Essentials of Theory and Practice. 1st Ames, IO: John Wiley & Sons, Ltd. 2012: 108-116.
- Watson A, Opresko D, Young R, Hauschild V, King J, Bakshi K. Organophosphate Nerve Agents. In: Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. London, UK: Academic Press; 2009:43-49.
- Woltjer RL. Neuropathologic Effects of Chemical Warfare Agents. In: Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. London, UK: Academic Press; 2009:653-659.