Signalment:
Gross Description:
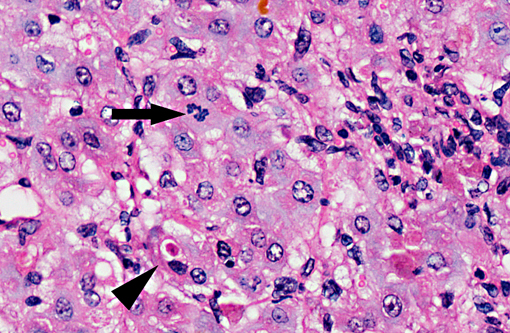
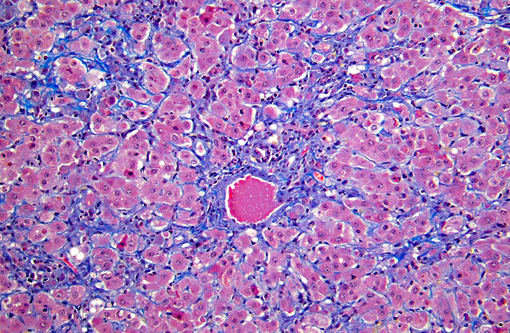
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Clinically, a variety of clinical syndromes are observed, dependent on the species involved, the dose of toxin and the duration of exposure.(2, 6, 8) In sheep, acute lupinosis occurs when animals graze highly toxic stubbles over a short period. Clinical signs include anorexia, lethargy, weakness and jaundice. Rarely, hepatic encephalopathy or photosensitization is observed. Clinical signs are typically seen within two days of introduction to stubble. Most animals within a flock are affected and deaths occur within three or four days.(7) Chronic lupinosis occurs following the ingestion of low doses of toxins over a prolonged period. Variable numbers of sheep within a flock are affected and animals are typically weak and in poor body condition. Jaundice may or may not be apparent, and those sheep that are jaundiced are commonly anemic. Mortality rates are low. The disease course may also be subacute and intermediate in severity, and it is this form of the disease that is most commonly observed in Australia.(8) A toxinrelated nutritional myopathy has also been reported in association with lupinosis, which may or may not respond to supplementation with vitamin E or selenium.(8) Experimental phomopsin toxicity has been shown to reduce reproductive performance also.(7)
On gross examination, livers from acutely affected sheep are enlarged and frequently discolored yellow or tan, with histological examination revealing variable degrees of hydropic change and an increased rate of hepatocyte loss (from widespread apoptosis). The degree of fatty change is variable and depends largely on the nutritional status of the animal. As the disease course progresses, increased, but ineffective mitotic activity becomes evident with the presence of numerous arrested mitoses. Progressive hepatic fibrosis occurs and there is accumulation of complex granular pigment within macrophages in the affected tissue, and may include any combination of copper, ferric iron, ceroid or lipofuscin. Variable degrees of portal hyperplasia may also be present.(6, 9) Some of these changes, including hepatic fibrosis and biliary hyperplasia, can also be observed in other hepatotoxicities including pyrrolizidine alkaloid and aflatoxicosis. As in phomopsin toxicity, these toxins inhibit normal mitosis, and DNA replication frequently continues resulting in the production of greatly enlarged hepatocytes (megalocytosis). In Australia, sheep grazing lupin stubble may also be concurrently exposed to plants containing pyrrolizidine alkaloids such as heliotrope (Heliotropium europium), which may result in additive or synergistic hepatotoxity.(2)
Lupinosis has been largely eliminated in Australia by the breeding of Phomopsis-resistant lupins, and is seen now in years with late finishes and/or increased summer rainfall.
JPC Diagnosis:
Conference Comment:
The contributor mentioned the possibility of photosensitization in acute phomopsin toxicosis. Photosensitization appears as erythema, dermal edema, vesicle or bullae formation, exudation and extensive epidermal necrosis. There are three types of photosensitization. Type I, or primary photosensitization, occurs from ingestion of a plant or drug containing photoreactive substances, which are deposited in the skin. Type II photosensitization occurs in animals with a genetic inability to metabolize heme pigments, which results in a buildup of the photoreactive hematoporphyrin pigments, such as uroporphyrin I, coproporphyrin I, and protoporphyrin III. Type III, or hepatogenous photosensitization, occurs with an abnormal buildup of phylloerythrin, which is a degradation product of chlorophyll, because of a damaged or immature liver. The photosensitization in this case is type III and is secondary to cholestasis(3). In cattle, phomopsin toxicity causes anorexia and ketosis in pregnant or lactating cows, and chronic exposure leads to fibrotic hepatitis with nodular regeneration (cirrhosis)9.
The toxic principles of true lupinosis (not to be confused with phomopsin toxicosis) are quinolizidine alkaloids, which causes nicotinic effects such as salivation, ataxia, seizures, and dyspnea, and the alkaloid anagyrine, which causes arthrogryposis in calves and lambs following in utero exposure(5, 10).
References:
2. Cheeke PR: Toxins in protein supplements and grain legumes. In: Natural Toxicants in Feeds, Forages, and Poisonous Plants, 2nd ed, pp. 163 193, 1998
3. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. vol. 1, 5th ed. Philadelphia, PA: Elsevier; 2007:623-6.
4. Kellerman TS, Coetzer JAW, Naud+�-� TW: Plant poisonings and Mycotoxicoses of Livestock in Southern Africa, pp. 27-29. Oxford University Press, Cape Town, 1988.
5. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. vol. 1, 5th ed. Philadelphia, PA: Elsevier; 2007:247.
6. Peterson JE, Jago MV, Payne AL, Stewart PL: The toxicity of phomopsin for sheep. Aust Vet J 64: 293 298, 1987
7. Radostits OM, Gay CC, Done SH, Blood DC, Hinchcliff KW, Constable PD: Diseases associated with toxins in plants, fungi, cyanobacteria, plant-associated bacteria, and venoms in ticks and vertebrate animals, 10th ed., pp.1906 1908. Saunders, Philadelphia, PA, 2007.
8. Seawright AA: Mycotoxins and Mycotoxicoses. In: Animal Health in Australia: Chemical and Plant Poisons, vol. 2, 2nd ed., pp. 173 177. Australian Government Publishing Service, Canberra, 1989
9. Stalker MJ, Hayes MA: Liver and Biliary System. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, vol. 2 ed. Maxie, MG, 5th ed., pp. 370-6. Elsevier, Philadelphia, PA, 2007
10. Thompson K. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. vol. 1, 5th ed. Philadelphia, PA: Elsevier; 2007:61.
11. Williamson PM, Highet AS, Gams W, Sivasithamparam K, Cowling WA: Diaporthe toxica sp. nov., the cause of lupinosis in sheep. Mycol Res 98: 1364 1368, 1994