Signalment:
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
As the term abiotrophy implies, the microscopic lesions are not considered the result of an acquired insult (e.g. infectious disease or intoxication), but rather is the consequence of an intrinsic metabolic disorder with a suspected hereditary basis of transmission. Besides this animal, two other meerkats from the same zoo (three and six-months of age) presented with similar clinicopathologic findings suggesting an inherited disease. These animals belong to a small colony, in which inbreeding is very common. No histologic evidence of an infectious disease was detected in the examined sections of all three animals.
Abiotrophy is characterized by the spontaneous degeneration and loss of neurons prematurely, and it is viewed as affecting the organ after it has developed its full cellular component.(2,10) This differs from hypoplasia, in which the cerebellum fails to form completely during development as the result of infectious diseases (e.g. feline panleukopenia, bovine viral diarrhea, classical swine fever), toxicities (e.g. organophosphate trichlorfon in piglets), and malnutrition (e.g. hypocuprosis in goat kids and lambs).(5)
Most commonly, animals with abiotrophy are neurologically normal at birth but will progressively develop cerebellar deficits in the postnatal period. However, some animal species may present a neonatal syndrome in which clinical signs are manifested in the immediate postnatal period (bovine and ovine) or can be delayed until time of ambulation (dog).(2,6) In postnatal syndromes, the onset and progression of clinical signs varies from a few days to months with a static course or slow progression.(3) Cerebellar ataxia, head tremor, truncal ataxia, symmetrical hypermetria, spacity, broad-based stance, and loss of balance are the most commonly described clinical manifestations in animals with cerebellar abiotrophy.(3) Besides visual and impaired proprioceptive positioning that were described by the field veterinarian, all the clinical signs evident in this meerkat are compatible with cerebellar disease.(3)
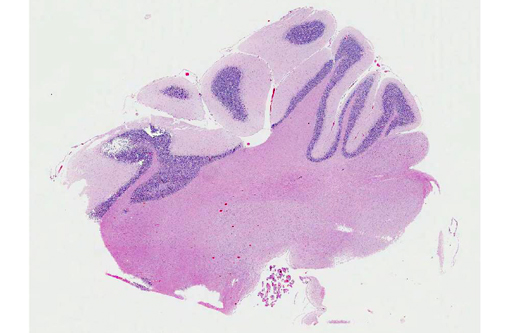
Grossly, the cerebellum can be normal or smaller, which is usually seen later in the course of the disease.(10) In animals that present gross changes of abiotrophy, cerebellar shrinkage can be noticeable with failure to fill the caudal part of the cranial vault, as well as with diminution of individual cerebellar folia, and broadening of sulci.(10) The involvement of the cerebellum is usually not uniform.(10) The cerebellum of the animal of this case was grossly unremarkable.
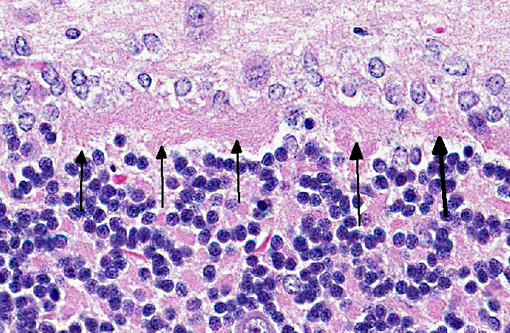
Microscopically, the distribution and characteristics of lesions vary depending on the species and breed of animals affected, but include: degeneration and loss of Purkinje cells, swelling of
Purkinje cell axons, astrogliosis, gliosis of cerebellar nuclei, Wallerian degeneration of the white matter of the folia, and spheroids.(6,10) Proliferation of Bergmann astrocytes is seen in folia where significant Purkinje cell loss has occurred.(10) Because the integrity of the granule cell neuron is dependent on its synaptic relationship with the dendritic zone of the Purkinje neuron, loss of the latter is followed by reduction of the granule cell neurons.(2) The animal in this case presented an apparent increase in the number of granular neurons that was evident when compared with the cerebellum of the age-matched control. The cause for this finding is undetermined. However, the other two meerkats that were diagnosed with cerebellar abiotrophy presented a decreased cellularity of the granular cell layer when compared with the control. Massive loss of Purkinje cells, which is accompanied by gliosis in the molecular layer, and atrophy of both molecular and granular layers are also features of poisoning in livestock that ingest several species of plants of the genus Solanum.(6) The consistency in the age of onset of clinical signs in these meerkats supported the diagnosis of abiotrophy. Extracerebellar lesions of abiotrophy have been described in the cerebellar cortex in the miniature Poodle, spinal Wallerian degeneration in rough-coated and Border Collies, and in Merino sheep.(6) The other sections of the CNS of all three meerkats were histologically normal.
JPC Diagnosis:
Conference Comment:
The contributor provides an excellent summary of cerebellar abiotrophy in various species of veterinary interest. Ruleouts for meerkat cerebellar abiotrophy include cerebellar hypoplasia due to in-utero/perinatal viral infection or toxin ingestion, neuroaxonal dystrophy and lysosomal storage diseases. Feline parvovirus, bovine pestivirus and ovine pestivirus have been shown to cause necrosis of the granular cell layer with resultant cerebellar hypoplasia in kittens, calves and lambs, respectively;(5) however, these viruses are not reported in meerkats. Additionally, when endogenous or exogenous factors such as infectious agents or toxins result in damage to fetal cerebellar components, the animal is typically affected at birth. On the other hand, with cerebellar abiotrophy the animal usually has normal cerebellar components at birth, but is subject to early-onset, hereditary, progressive cerebellar degeneration postnatally,(8) although as noted by the contributor there are exceptions to this generalization. Neuroaxonal dystrophy, reported in dogs, cats, horses and sheep, is a degenerative condition that occasionally affects the cerebellum and is characterized by nerve fiber degeneration and formation of large spheroids.(1,8) Lysosomal storage diseases occur when a lack of specific lysosomal enzymes causes various materials to accumulate in nerve cells and macrophages.(8) Neuroaxonal dystrophy and lysosomal storage diseases have not been reported in meerkats.
References:
2. de Lahunta A. Abiotrophy in domestic animals: a review. Can J Vet Res. 1990;54:65-76.
3. de Lahunta A, Glass E. Cerebellum. In: de Lahunta A, Glass E. eds. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St. Louis, MO: Saunders Elsevier; 2009:348-388.
4. Juan-Salles C, Prats N, Lopez S, Domingo M, Marco AJ, Moran JF. Epizootic disseminated toxoplasmosis in captive slender-tailed meerkats (Suricata suricatta). Vet Pathol. 1997;34:1-7.
5. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 1. Philadelphia, PA: Elsevier; 2007:281-487.
6. Zachary JF. Central nervous system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Mosby Elsevier; 2007:833-953.
7. Mouser P, Levy M, Sojka JE, Ramos-Vara JA. Cerebellar abiotrophy in an alpaca (Lama pacos). Vet Pathol. 2009;46:1133-1137.
8. Sato J, Sasaki S, Yamada N, Tsuchitani M. Hereditary cerebellar degenerative disease (cerebellar cortical abiotrophy) in rabbits. Vet Pathol. 2012;49(4):621-628.
9. Sladky KK, Dalldorf FG, Steinberg H, Wright JF, Loomis MR. Cholesterol granulomas in three meerkats (Suricata suricatta). Vet Pathol. 2000;37:684-686.
10. Summers BA, Cummings JF, de Lahunta A. Degenerative diseases of the central nervous system. In: Summers BA, Cummings JF, de Lahunta A, eds. Veterinary Neuropathology. 1st ed. St. Louis, MO: Mosby-Yearbook, Inc; 1995:300-307.
11. Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anatomical Science International. 2002;77:94-108.