Signalment:
Eight-month-old, Dorset ewe, (
Ovis aries).This ewe was
enrolled in a research study in which it underwent surgery and MRI the
following day, both under general anesthesia. The ewe initially recovered
normally, displaying brief weakness in the hindlimbs immediately after
surgery. However, over the next two weeks, it gradually became dull and
lethargic, dehydrated, inappetent, and weakened despite supportive care. The
animal was found recumbent, hypothermic, tachycardic, and dyspneic 13 days
after surgery and died before it was able to be euthanized.
Gross Description:
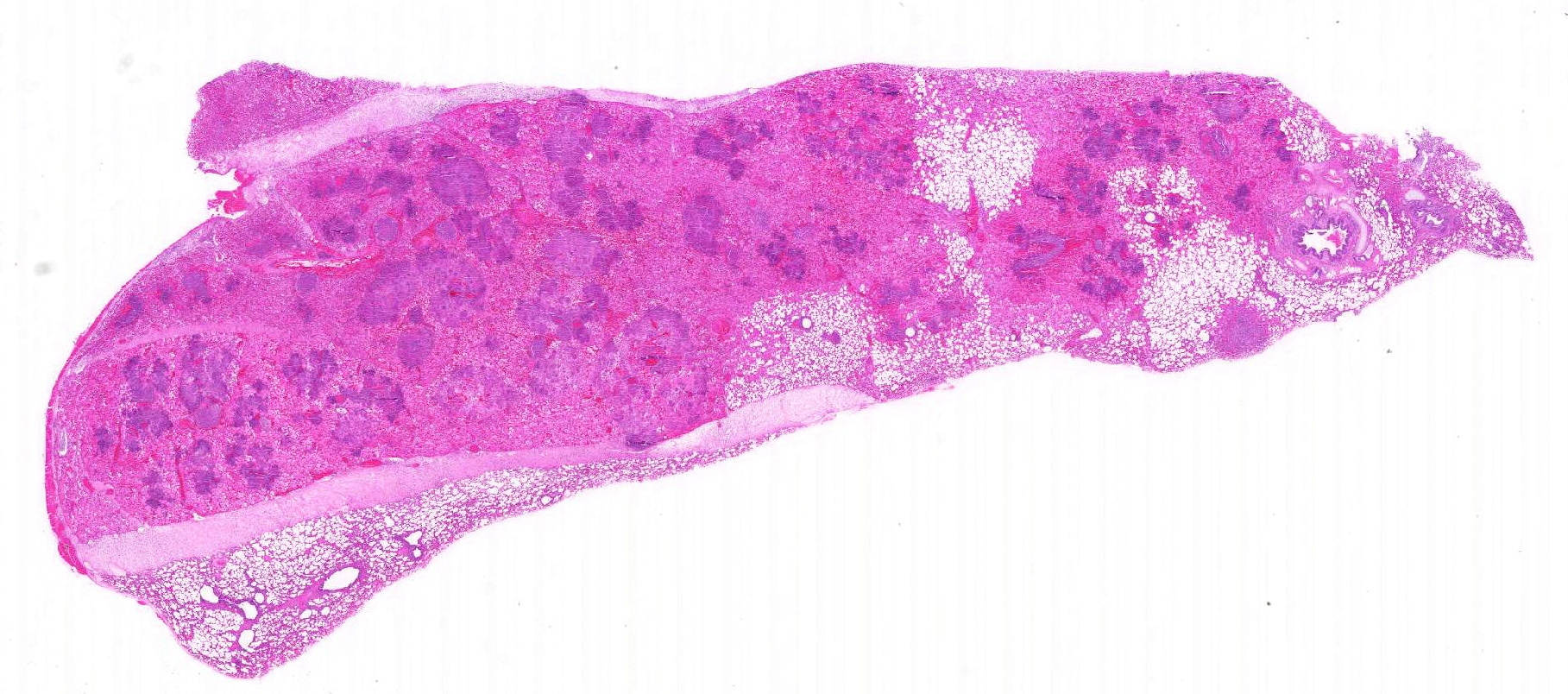
The
major gross lesion was in the lungs. The right cranial lung lobe was half
normal size, mottled dark red to black, and firm on palpation. The cranial
aspect of the right middle lung lobe appeared similarly. Dissection of the
bronchial tree did not reveal the presence of foreign material. Additionally,
several ulcers, up to 1 x 2 cm, were present in the trachea.
Histopathologic Description:
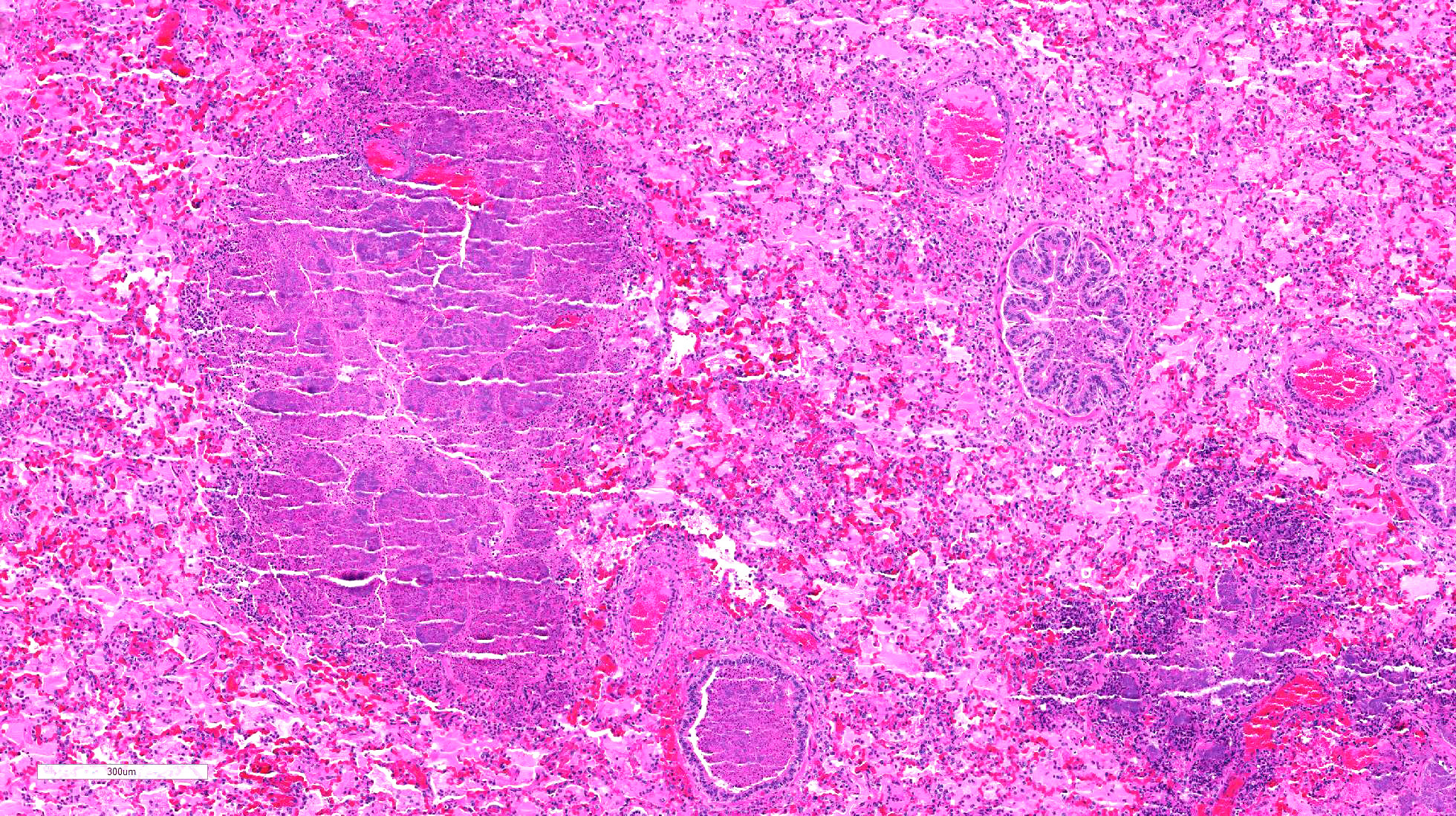
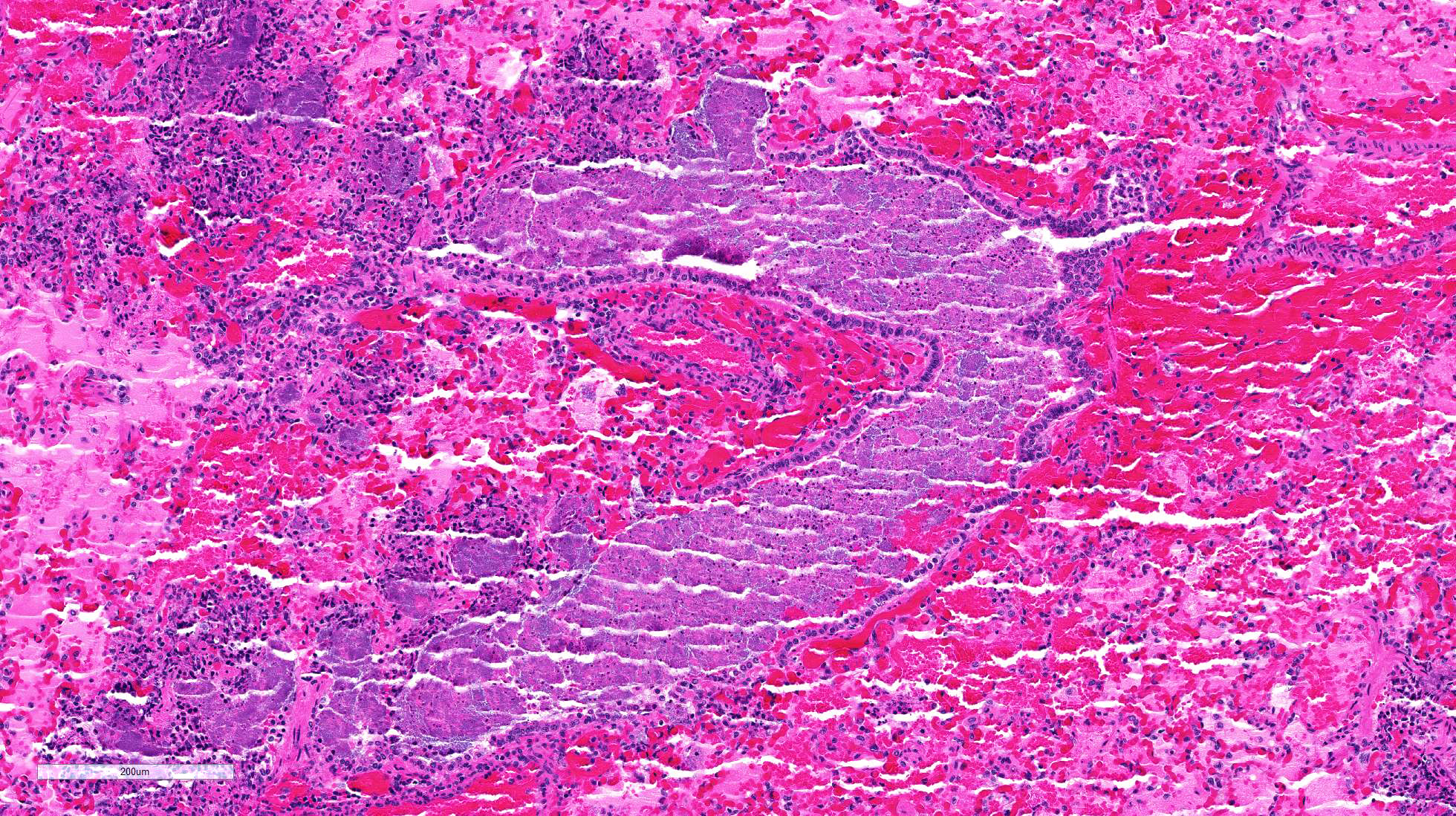
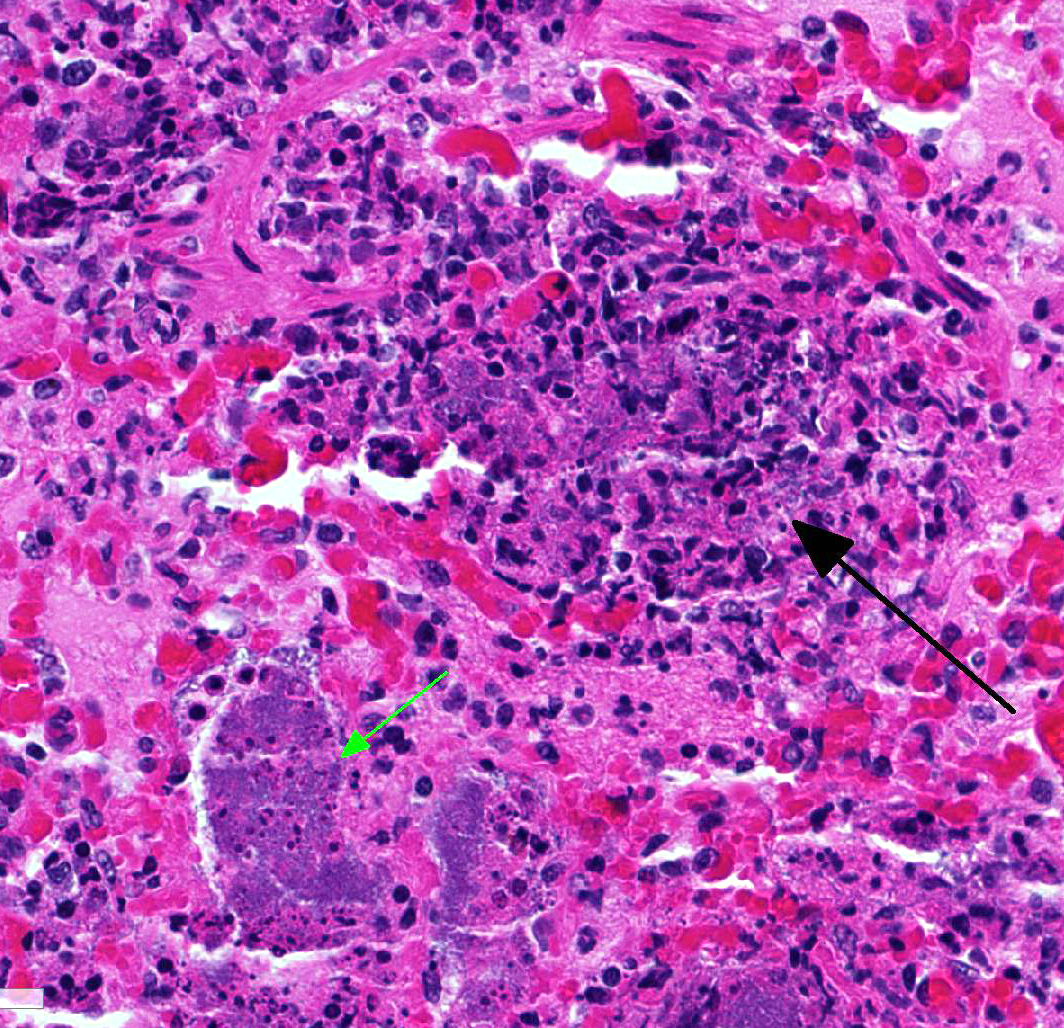
The
lung is markedly hypercellular, owing to filling of alveoli by degenerate
inflammatory cells, amorphous and fibrillar eosinophilic material (edema and
fibrin, respectively), basophilic streaming nuclear and eosinophilic
cytoplasmic debris, and myriad gram-positive bacterial rods. In some regions,
well-defined areas with architectural preservation without cellular detail
(coagulative necrosis) are present. Bronchi and bronchiole lumina frequently
contain abundant bacteria and sloughed bronchial epithelium. Interlobular septa
are thickened up to 2mm wide by fibrin and edema, and blood vessels were
congested and contained bacteria. The pleura is expanded up to 3x normal with
blood.
Morphologic Diagnosis:
Broncho-pneumonia, multifocal, subacute, severe,
necrotizing.
Lab Results:
Bacterial
culture of the lung yielded a pure culture of
Trueperella pyogenes.
Condition:
Choriomeningoencephalitis/Borrelia burgdorferi
Contributor Comment:
All findings in
this case were consistent with bronchopneumonia caused by
Trueperella
pyogenes (formerly
Arcanobacterium pyogenes), a gram-positive
non-motile, non-sporeforming, short, rod-shaped bacterium.
6 Trueperella
pyogenes is one of the most common opportunistic pathogens in domestic
livestock and is often commensal flora in the mammary gland, upper respiratory,
urogenital, and gastrointestinal tracts.
6,10 Although
T. pyogenes
can act as a primary pulmonary pathogen, infection usually follows physical or
microbial trauma which overcomes the pulmonary defense mechanisms, allowing for
colonization of the lungs.
1,8 The pneumonia was initially suspected
to be due to aspiration shortly after recovery from anesthesia, but foodstuff
was not present in the respiratory tract. The reason for the infection was
likely due to a combination of stressors, including what was interpreted as
intubation-associated ulceration of the trachea. Trueperella
pyogenes produces
and utilizes a variety of virulence factors to colonize, damage, and persist
within a variety of tissues in the host. The most important factor is pyolysin
(PLO), a cytolysin, which is able to bind to and create pores in the cell
membranes of erythrocytes, polymorphonuclear cells, and macrophages resulting
in cell lysis.
6 Trueperella pyogenes mutants lacking the PLO
gene were unable to cause an intraperitoneal infection in mice injected with 10
8
bacteria, whereas replacement of the PLO gene to the
T. pyogenes
mutants restored full virulence.
5 Additional virulence factors used
by
T. pyogenes include fimbriae
13, extracellular matrix
binding proteins specific for collagen
4, fibrinogen, fibronectin
5,
and exoenzymes including DNases
11, proteases
12, and
neuraminidases
7 which degrade host nucleic acids, proteins, and acid residues, respectively.
Note:
Multiple different tissue sections of lung were used for the slides submission;
therefore, not all the participants will receive similar serial microslide
sections.
JPC Diagnosis:
Lung: Bronchopneumonia, necrotizing and fibrinosuppurative,
multifocal to coalescing, severe, with marked alveolar and septal edema and
numerous large colonies of bacilli, Dorset sheep,
Ovis aries.
Conference Comment:
This case demonstrates the
characteristic gross and histologic lesions associated with bacterial
bronchopneumonia. Conference participants described the suppurative
inflammation filling bronchi and bronchioles surrounded by multifocal to
coalescing areas of necrosis, and readily identified numerous large colonies of
bacilli within the areas of inflammation. Participants discussed the
differential diagnosis for large colony forming bacteria in tissue section to
include:
Yersinia sp,
Actinomyces sp.,
Actinobacillus sp.,
Corynebacterium sp,
Staphylococcus sp.,
Streptococcus sp.,
and
T. pyogenes.
Trueperella pyogenes is one of the most common
opportunistic pathogens present on the mucosal surfaces of domestic animals.
1,2,5,8
The bacterium induces suppurative inflammation within a wide variety of organs
and is an important cause of abortion, arthritis, endocarditis, mastitis,
osteomyelitis, and pneumonia resulting in significant losses in production
animals.
2,4 Additionally,
T. pyogenes is widespread in the
wild animal population and has been reported as an important cause of cerebral
abscesses in young male white-tailed deer as a consequence of antler
development and conspecific aggression between bucks. Mortality can reach up to
35% in affected free-ranging adult male deer.
3
As
mentioned by the contributor, while
T. pyogenes can be a primary
pathogen, it is usually associated with physiologic trauma to a mucosal
membrane, concurrent primary infection, or immune suppression.
1,2,3,8
As postulated by the contributor in this case, there well may be an association
with placement of the endotracheal tube. Other common commensal organisms of
the ruminant upper respiratory tract that can cause opportunistic
bronchopneumonia include
Mannheimia haemolyica,
Pasteurella multocida,
and
Bibersteinia trehalosi. Causes of primary infectious pneumonia in
sheep include parainfluenza virus 3, respiratory syncytial virus, and
Bordetella
parapertussis, which can predispose sheep to secondary infection by the
commensal bacteria mentioned above.
1,3,8 Mycoplasma ovipneumoniae is
another important primary etiologic agent involved in chronic enzootic
pneumonia; also known as chronic non-progressive pneumonia, it is a
multi-factorial disease complex affecting lambs less than one-year-old. Mycoplasma
ovipneumoniae usually causes a mild, subclinical infection that results in
poor growth unless complicated by stressors such as over-crowding, inclement
weather, or poor air quality.1,2,3,8
Another primary cause of pneumonia in sheep includes the
maedi-visna virus, which results in lifelong persistent viral infection and
leads to ovine progressive pneumonia (OPP), encephalitis, arthritis, and
mastitis in sheep. This lentivirus is a member of the family Retroviridae,
and is closely related to caprine arthritis-encephalitis virus. First reported
in Iceland, the maedi (respiratory form) occurs in sheep older than three
years, while the visna (neurologic form) occurs in younger sheep.1,9
The respiratory form is characterized by interstitial pneumonia with prominent
perivascular and peribronchial lymphoid nodules. This slowly progressive
pneumonia is often complicated by secondary bacterial infection, especially T.
pyogenes.1 Peste des petits ruminants (PPRV), a morbillivirus,
has also been reported to cause primary respiratory disease in small ruminants
in Africa and parts of Asia. This virus primarily affects the cranioventral
lung lobes and causes a bronchointerstitial pneumonia.1,8,9
References:
1. Caswell JL, Williams KJ. Respiratory system. In:
Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 6th
ed. Vol. 2. Philadelphia, PA: Elsevier; 2016:557-560.
2. Cohen BS, Belser EH, et al. Isolation and genotypic
characterization of Trueperella (Arcanobacterium) pyogenes
recovered from active cranial abscess infections of male white-tailed deer
(Odocoileus virginianus). J Zoo Wildl Med. 2015; 46(1):62-67.
3. Dassanayake RP, Shanthalingam S, Herndon CN, et al. Mycoplasma
ovipneumoniae can predispose bighorn sheep to fatal Mannheimia
haemolyticapneumonia. Vet Microbiol. 2010; 145:354-359.
4. Esmay
PA, et al. The Arcano-bacterium pyogenes collagen-binding protein, CbpA,
promotes adhesion to host cells. Infect Immun. 2003;
71(8):4368-74.
5. Jost BH, Billington SJ. Arcano-bacterium pyogenes:
Molecular pathogenesis of an animal opportunist. Antonie Van
Leeuwenhoek. 2005; 88(2):87-102.
6. Jost BH, Songer JG, Billington SJ. An Arcanobacterium
(Actinomyces) pyogenes mutant deficient in production of the pore-forming
cytolysin pyolysin has reduced virulence. Infect Immun. 1999;
67(4):1723-8.
7. Jost BH, Songer JG, Billington SJ. Cloning, expression, and
characterization of a neuraminidase gene from Arcanobacterium pyogenes. Infect
Immun. 2001; 69(7):4430-7.
8. Lopez A, Martinson SA. Respiratory system, mediastinum, and
pleurae. In: Pathologic Basis of Veterinary Disease. Zachary JM ed. 6th
ed. St. Louis: Elsevier; 2017:537-540.
9. MacLachlan NJ, Dubovi EJ, eds. Fenners
Veterinary Virology. 4th
ed. London, UK: Elsevier; 2011:267-268,308-323.
10. Queen
C, Ward AC, Hunter DL. Bacteria isolated from nasal and tonsillar samples of
clinically healthy Rocky Mountain bighorn and domestic sheep. J Wildl
Dis. 1994; 30(1):1-7.
11. Ramos,
CP, Foster G, Collins MD. Phylogenetic analysis of the genus Actinomyces based
on 16S rRNA gene sequences: description of Arcanobacterium phocae sp.
nov., Arcanobacterium bernardiae comb. nov., and Arcanobacterium
pyogenes comb. nov. Int J Syst Bacteriol, 1997; 47(1):46-53.
12. Takeuchi
S, Kaidoh T, Azuma R, Assay of proteases from Actinomyces pyogenes isolated
from pigs and cows by zymography. J Vet Med Sci. 1995;
57(5):977-9.
13. Yanagawa
R, Honda E Presence of pili in species of human and animal parasites and
pathogens of the genus Corynebacterium. Infect Immun. 1976;
13(4):1293-5.