CASE IV: L20 163 (JPC 4152938)
Signalment:
7-year-old, spayed female, Labrador Retriever, domestic dog (Canis lupus familiaris)
History:
The dog had a history of anorexia, progressive weakness, and lameness of undetermined duration. On presentation to the Louisiana State University Veterinary Teaching Hospital, the dog was depressed but responsive, with tachypnea and tachycardia. On thoracic radiographs, a lytic lesion at the dorsal border of the right scapula was noted as well as a right caudodorsal lung mass/consolidation. Abdominal ultrasound showed numerous targetoid lesions in the spleen as well as a right caudodorsal lung mass. Due to the poor prognosis, humane euthanasia was elected.
Gross Pathology:
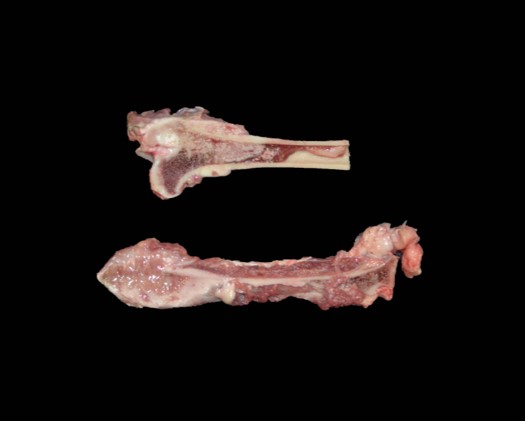
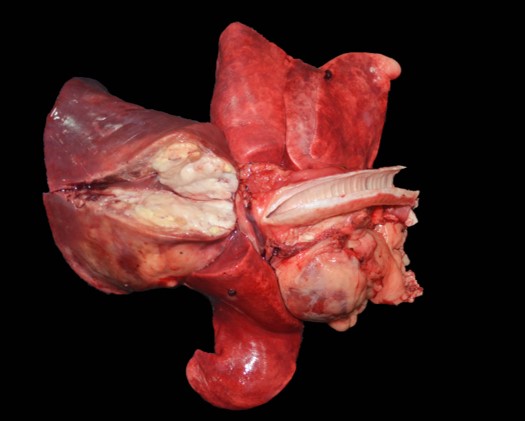
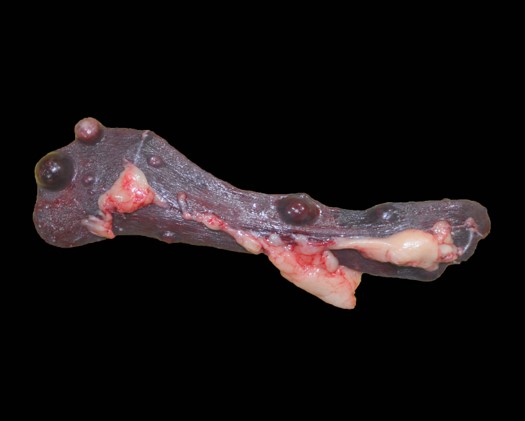
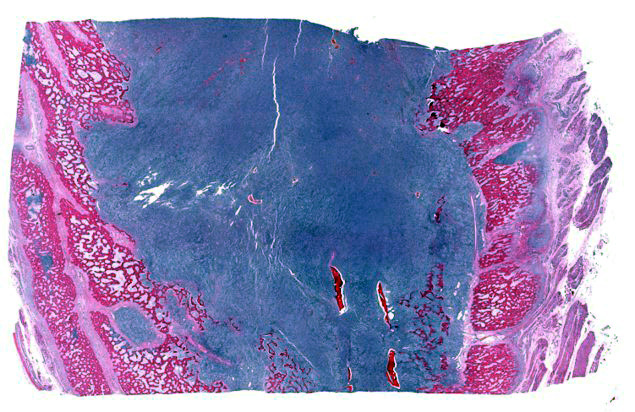
The dorsocranial aspect of the right scapula was expanded by a 6.0 x 7.5 x 0.3 cm mass that extended to the dorsocaudal aspect of the bone. On the cut surface, the affected scapula was replaced by an infiltrative, ill-defined, soft and tan mass that filled the adjacent marrow spaces and was delimited by abundant, superficial cortical bone/ periosteum. The right femur had the proximal aspect of the metaphysis adjacent to the femoral neck expanded by a focal, ill-defined area of periosteal thickening measuring approximately 2.0 x 1.0 x 0.4 cm; on the cut surface, the intralesional marrow spaces were filled with soft and tan tissue similar to that noted in the right scapula. The parenchyma of the right caudal lung lobe was extensively effaced by a well-demarcated, multilobular, firm and tan mass that measured 8.0 x 7.5 x 6.0 cm and slightly bulged on the cut surface. In the heart, the endocardial surface of the interventricular septum had a focal, white, and slightly firm nodule that measured 0.5 x 0.3 x 0.4 cm and protruded into the left ventricle. In the spleen, multiple round, soft, mottled tan to dark red and variably sized nodules (ranging in size from 0.2 x 0.2 x 0.1 cm to 1.5 x 1.5 x 1.5 cm) were noted throughout both the visceral and parietal surfaces.
Laboratory Results:
- Complete Blood Count:
o Mean corpuscular value (MCV): 59.9 fL (62-77 fL)
o Red cell distribution width (RDW): 17% (12-14%)
o White blood cell count (WBC): 23.3 x 103/μl (8-14.5 x 103/μl)
o Segmented neutrophils (absolute value): 18.8 x 103/μl (3-11.5 x 103/μl)
o Lymphocytes (absolute value): 0.70 x 103/μl (1-4.8 x 103/μl)
o Monocytes (absolute value): 3.7 x 103/μl (0.1-1.4 x 103/μl)
No significant abnormalities were identified in the biochemical profile.
Cytology:
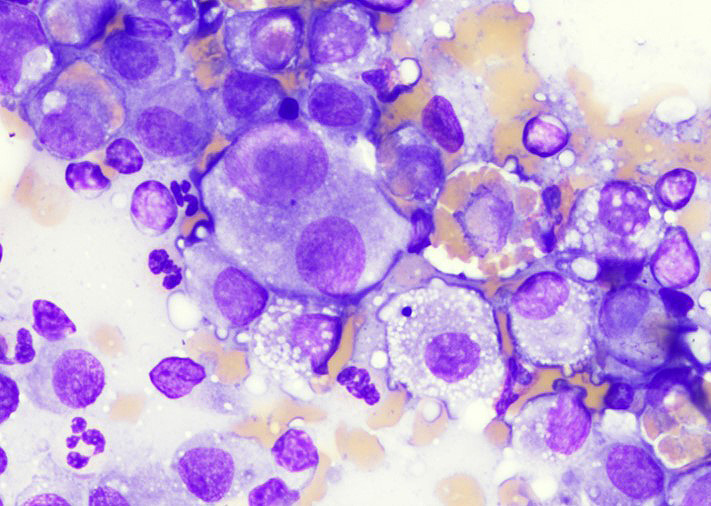
Scapula: Four smears with overall low cellularity were examined. Rare individualized, rounded to elongated multinucleated cells with abundant basophilic cytoplasm containing few small, discrete vacuoles and round to ovoid nucleus (10-12 μm in diameter) with finely stippled chromatin and a single small, prominent nucleoli were noted. No infectious agents were seen.
Spleen and lung: Multiple smears from each location were evaluated. Round to stellate neoplastic cells with variably sized nuclei, small discrete intracytoplasmic vacuoles and occasional erythrophagocytosis were observed along with numerous binucleated to multinucleated cells.
Microscopic Description:
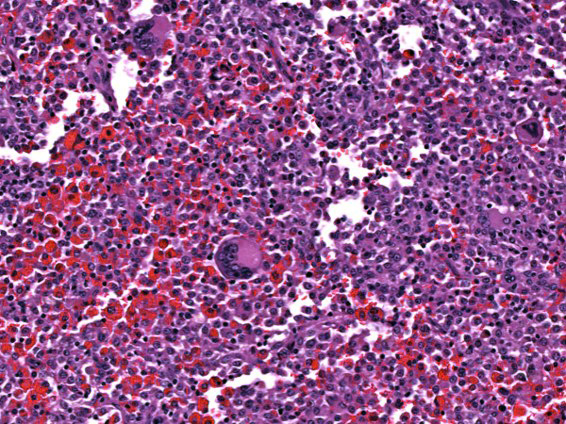
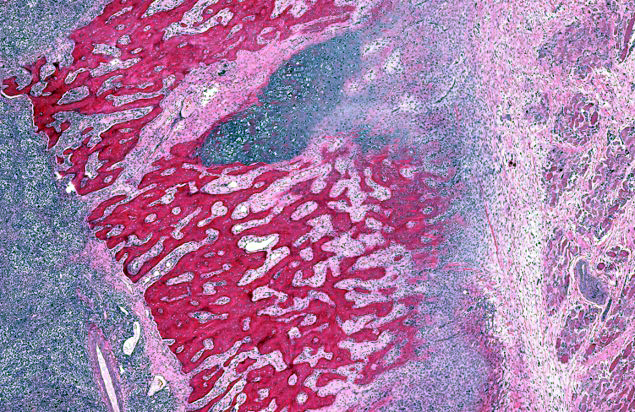
Bone (right scapula): The normal architecture of the bone is extensively effaced by a non-encapsulated, infiltrative, and densely cellular neoplasm composed of sheets of neoplastic round to spindle cells supported by scant pre-existing stroma. Neoplastic cells are pleomorphic, are often large, and have distinct cell borders, with moderate to abundant eosinophilic cytoplasm, and a round to oval central nucleus with coarsely stippled chromatin and one to two prominent nucleoli. Anisocytosis and anisokaryosis are severe, with frequent giant and multinucleated neoplastic cells with bizarre nuclei. Nine atypical mitotic figures are counted in 10 high power fields. Occasionally, neoplastic cells contain intracytoplasmic erythrocytes (erythrophagocytosis) and intracytoplasmic hemosiderin pigment. At the periphery, the surface of the bone is irregularly expanded by proliferation of new trabecular bone, multifocal islands of partially ossified hyaline cartilage, marrow spaces filled with either neoplastic round cells or loose fibrous connective tissue, and markedly thickened periosteum composed of numerous proliferating osteoblasts and fibroblasts. The peripheral skeletal muscle is composed of multifocal shrunken myofibers (atrophy).
Histologic changes in other tissues (not submitted):
The splenic parenchyma is multifocally expanded by variably sized neoplastic nodules composed of similar neoplastic cells as described in the scapula, with frequent bizarre nuclei and occasional erythrophagocytosis. Similarly affected tissues include the lungs, heart, adrenal glands, pancreas, and proximal right femur.
Immunohistochemistry:
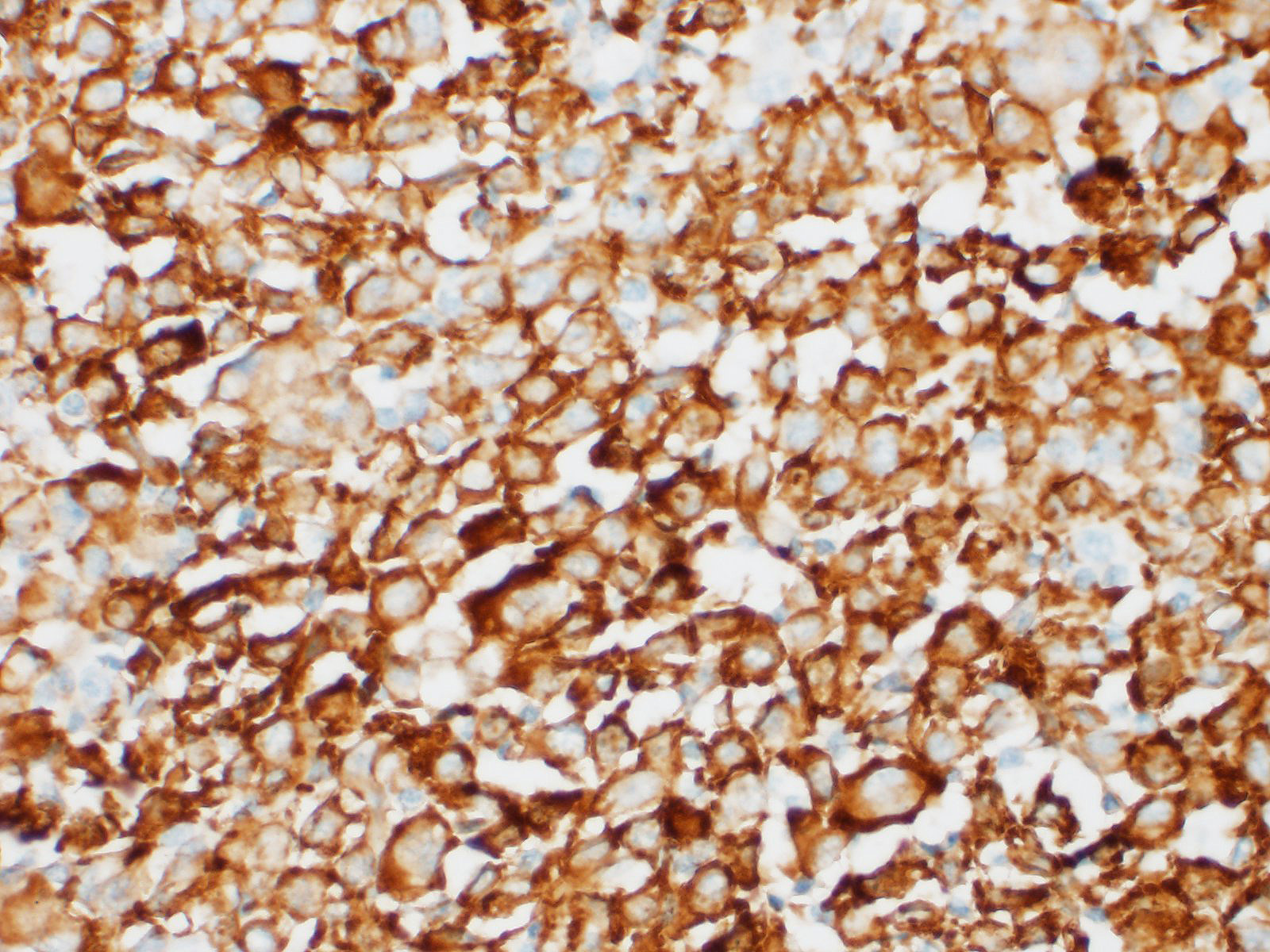
Anti-Iba-1 and anti-CD11d-specific immunohistochemistry were performed on sections of spleen. Neoplastic cells had strong cytoplasmic immunostaining for Iba-1 but did not have any immunostaining for CD11d.
Contributor's Morphologic Diagnoses:
Bone (right scapula): Histiocytic sarcoma, disseminated (based on multiple tissues examined).
Contributor's Comment:
Canine proliferative histiocytic disorders include benign (histiocytoma) and malignant (e.g. histiocytic sarcoma [HS] complex) neoplastic as well as nonneoplastic diseases (e.g. cutaneous and systemic [reactive] histiocytosis). Among the entities grouped within the histiocytic sarcoma complex, the localized and disseminated forms are proliferative disorders originating from interstitial dendritic cells, while the hemophagocytic histiocytic sarcoma is a distinctive entity arising from bone marrow-derived macrophages, mostly within the splenic red pulp or bone marrow.1-3,6,9-13,15 Canine HS may initially present either as a localized, solitary tumor or as a disseminated, multicentric disease. Metastatic disease is
frequently observed as the disease progresses.2,9,10,15
|
Disease |
Cell of Origin |
Immunophenotype |
|
Histiocytoma |
Langerhans cell |
CD1a+, CD11c/CD18+, E-cadherin+, CD204-, Iba1+ |
|
Cutaneous Langerhans cell histiocytosis |
Langerhans cell |
CD1a+, CD11c/CD18+, E-cadherin+, CD204-, Iba1+ |
|
Histiocytic sarcoma |
Interstitial dendritic cell |
CD1a+, CD11c/CD18+, CD204+/-, Iba1+ |
|
Histiocytic sarcoma, hemophagocytic |
Macrophage |
CD1a+/-, CD11d/CD18+, CD204+, Iba1+ |
|
Cutaneous histiocytosis |
Activated interstitial dendritic cell |
CD1a+, CD4+, CD11c/CD18+, CD90+, CD204-, Iba1+ |
|
Systemic histiocytosis |
Activated interstitial dendritic cell |
CD1a+, CD4+, CD11c/CD18+, CD90+, Iba1+ |
|
Table 1. Immunophenotypic differentiation of canine histiocytic diseases (From Tumors of Domestic Animals [2016], Chapter 8, Canine and Feline Histiocytic Diseases by Peter F. Moore). |
||
The clinical presentation in dogs affected by HS is highly variable, depending on the organ systems affected.10,15 Clinical signs can therefore include anorexia, weight loss, lethargy, dyspnea, lameness, neurological signs, among others.
HS most commonly affects the spleen, lungs, bone marrow, lymph nodes, skin and subcutis, joints, and central nervous system. The masses are often destructive and composed of large round to spindle neoplastic cells with ovoid to reniform nuclei and abundant eosinophilic cytoplasm with marked atypia and variably sized nuclei with multinucleation and frequent atypical mitosis.3,10 In this particular case, neoplastic cells exhibiting erythrophagocytosis were observed both cytologically and histologically, resembling a hemophagocytic HS, which was initially considered as the presumptive diagnosis; however, immunohistochemical characterization of neoplastic cells revealed lack of expression of CD11d, an integrin that has been shown to distinctively characterize this hemophagocytic form.15 Hemophagocytic HS is reported in similar canine breeds as histiocytic sarcoma, namely Bernese Mountain dogs, Golden Retrievers, Labrador Retrievers, Flat-coated Retrievers, and Rottweilers. Clinicopathological abnormalities are characterized by rapidly progressive, regenerative hemolytic anemia and thrombocytopenia with negative Coombs test, hypoalbuminemia, and hypocholesterolemia (not present in this particular case).10,15 Macroscopically, hemophagocytic HS does not form mass lesions within primary sites (spleen and bone marrow) as opposed to histiocytic sarcoma, but causes a diffuse enlargement of the spleen instead.10
Disseminated HS is often difficult to grossly differentiate from systemic reactive histiocytosis,10,11 a systemic disorder primarily affecting Bernese Mountain dogs, characterized by lesions in the skin and internal organs (such as lung, liver, spleen, kidneys, and bone marrow). Histologically, reactive histiocytosis is characterized by vasocentric and often vasodestructive proliferation of large, mildly atypical histiocytes. Cells with bizarre or multiple nuclei are lacking, however, in contrast to HS.
Differential diagnoses for tumors with high nuclear atypia include the following:
- Anaplastic lymphoma
- High grade/Grade III mast cell tumor
- Poorly differentiated carcinoma
- Amelanotic melanoma
- Osteosarcoma
- Undifferentiated sarcomas
Immunohistochemistry using specific antibodies for monocyte/macrophage/ dendritic cell lineage, B and T cell lineage, and other cells (e.g. epithelial cells or melanocytes) is often imperative to reach a definitive diagnosis (Table 1).9,10,15
Although histiocytic sarcoma occurs mainly in dogs and cats, other animals such as cows, mice and some birds can develop this tumor as well; however, their occurrence is rare.4,8,10,13
Contributing Institution:
Louisiana Animal Disease Diagnostic Laboratory (LADDL) and Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University (http://www1.vetmed.lsu.edu/laddl/index.html and https://www.lsu.edu/vetmed/pbs/index.php)
JPC Diagnosis:
Bone: Histiocytic sarcoma.
JPC Comment:
The contributor provides an excellent overview of canine proliferative histiocytic disorders, a diverse complement of benign and malignant entities originating from various histiocytic linages that may arise in and affect various organ systems.
Multiple dog breeds have demonstrated an increased incidence of histiocytic sarcoma (HS), including Bernese mountain dogs, flat coated retrievers, golden retrievers, and Rottweilers, while other breeds are also sporadically affected. Bernese mountain dogs
are particularly predisposed and are estimated to be 225 times more likely to develop HS compared to other breeds, with up to 25% of individuals being affected in their lifetime.14
A recent report5 describing a genome-wide association study (GWAS) using Bernese mountain dog DNA from 172 HS and 128 control cases confirmed previous reports that the main locus associated with HS development in this breed is the MTAP-CDK2A region on chromosome #11 (CFA11/HSA9q21). An additional single nucleotide variation associated with HS was identified on chromosome #20 at an intron of FHIT (a tumor suppressor gene), while a suspected locus was also identified on chromosome #5 in an intron of the SPNS3 gene. SPNS3's function is largely unknown, however, its paralogous gene (SPNS2) has a role in immunological development and has been associated with inflammatory and autoimmune disease in addition to defective macrophage phagocytosis. Interestingly, each of these three regions have previously been associated with other malignancies including osteosarcoma (CDK2A), lymphoma and hemangiosarcoma (SPNS3), and mast cell tumors (FHIT).5
In addition, multiple studies have found dogs with a history of orthopedic disease are at increased risk of developing periarticular HS, the most common synovial neoplasm in dogs. One study found Bernese mountain dogs are five times more likely to develop periarticular HS in a diseased joint compared to a healthy joint. Although this phenomenon occurs in breeds classically predisposed to HS, it does not appear to be restricted to only those breeds. One retrospective study7 reviewing 28 canine patients from nine breeds with periarticular HS found those with a history of prior joint disease were over 13 times more likely to develop periarticular HS compared to the control group. Even more striking, history of joint disease in the stifle joint was associated 64 times greater risk of developing periarticular HS when affected joints were compared independently. Rottweilers were found to be the most commonly affected when breeds were assessed independently.7
The pathogenesis involved in the development of HS as the result of prior joint disease is unknown. However, inflammation likely plays a role as increased numbers of antigen presenting cells are present in synovial membranes of dogs with cruciate disease, degenerative joint disease, and rheumatoid arthritis. In regard to cruciate disease specifically, synovitis has been identified as risk factor for future rupture of the cruciate ligament, potentially due to weakening as a result of degradation by inflammatory mediators. In addition, synovitis and osteoarthritis are known to persist following surgical repair of cruciate injuries. Therefore, breeds predisposed to cruciate injuries, such as Rottweilers, may inherently be predisposed to the development of periarticular HS.7 Interestingly, administration of anti-inflammatory medications has been found to reduce the risk of HS in Bernese mountain dogs, further supporting the theory in regard to inflammation's role in the development of periarticular HS.7,14
References:
1. Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol 2002; 39(1): 74?83.
2. Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: An overview. Can Vet J 2007; 48(10): 1041?1050.
3. Glick AD, Holscher M, Campbell GR. Canine cutaneous histiocytoma: Ultrastructural and cytochemical observations. Vet Pathol 1976; 13:374?380.
4. Hao X, Fredrickson TN, Chattopadhyay SK, Han W, Qi CF, Wang Z, Ward JM, Hartley JW, Morse III HC. The histopathologic and molecular basis for the diagnosis of histiocytic sarcoma and histiocyte-associated lymphoma of mice. Vet Pathol 2010;47(3): 434-445.
5. Hédan B, Cadieu É, Rimbault M, et al. Identification of common predisposing loci to hematopoietic cancers in four dog breeds. PLoS Genet. 2021;17(4):e1009395.
6. Kraje AC, Patton CS, Edwards DF. Malignant histiocytosis in 3 cats. J Vet Intern Med. 2001; 15(1): 252?256.
7. Manor EK, Craig LE, Sun X, Cannon CM. Prior joint disease is associated with increased risk of periarticular histiocytic sarcoma in dogs. Vet Comp Oncol. 2018;16(1):E83-E88.
8. Matsuda K, Nomoto H, Kawamura Y, Someya Y, Koiwa M, Taniyama H. Hemophagocytic histiocytic sarcoma in a Japanese black cow. Vet Pathol 2010; 47(2): 339-342.
9. Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol 2014; 51(1): 167-184.
10. Moore PF. Canine and feline histiocytic diseases. In: Meuten DJ. Tumors in Domestic Animals. 5th edition Ames, Iowa: Iowa State Press; 2017: 322-335.
11. Moore PF. The UC Davis Canine Histiocytosis site. Histiocytic sarcoma and malignant histiocytosis. Available from: http://www.histiocytosis.ucdavis.edu/ Last accessed March 23, 2020.
12. Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: A proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006; 43(5): 632-645.
13. Pandiri AR, Gimeno IM, Reed WM, Fitzgerald SD, Fadly AM. Soubgroup J avian leukocytosis virus-induced histiocytic sarcomatosis occurs only in persistently viremic but not immunotolerized meat-type chickens. Vet Pathol 2009; 46(2): 282-287.
14. Ruple A, Morley PS. Risk Factors Associated with Development of Histiocytic Sarcoma in Bernese Mountain Dogs. J Vet Intern Med. 2016;30(4):1197-1203.
15. Valli T, Kiupel M, Bienzle D. Hematopoietic system. In: Maxie MG ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 1. 6th ed. St. Louis, MO: Elsevier; 2016: 243-254.