CASE IV:
Signalment:
10 day-old, female, Standardbred horse, Equus caballus, equine
History:
In the late spring of 2023, five, 3-4 day old foals in a Standardbred breeding farm devel-oped clinical signs of pneumonia over a peri-od of several weeks. Four of these foals did not respond to antibiotic treatment and died at approximately 8-10 days of age. Two of these foals were necropsied and both exhibit-ed severe acute interstitial pneumonia and severe edema, with microscopic features most consistent with acute diffuse alveolar dam-age. Equine herpesviruses 1 and 4, and eq-uine influenza type A were not detected in PCR testing of lungs samples from both foals. No clinical abnormalities were noted in the dams of these foals, or in any other horses on the farm.
The last of these foals (the 5th one reported) was noted to be lethargic and tachypneic at 4 days of age. This filly was treated with ceft-iofur sodium, gentamicin, flunixin meglu-mine, dexamethasone, furosemide, and oxy-gen therapy. Radiographs of the thorax re-vealed abnormalities consistent with intersti-tial pneumonia. Despite treatment, the foal?s condition progressively worsened until she suddenly decompensated and began agonal breathing early morning, 6 days after clinical signs were first noted. The filly was humane-ly euthanized with IV Euthanyl and submit-ted for necropsy.
Gross Pathology:
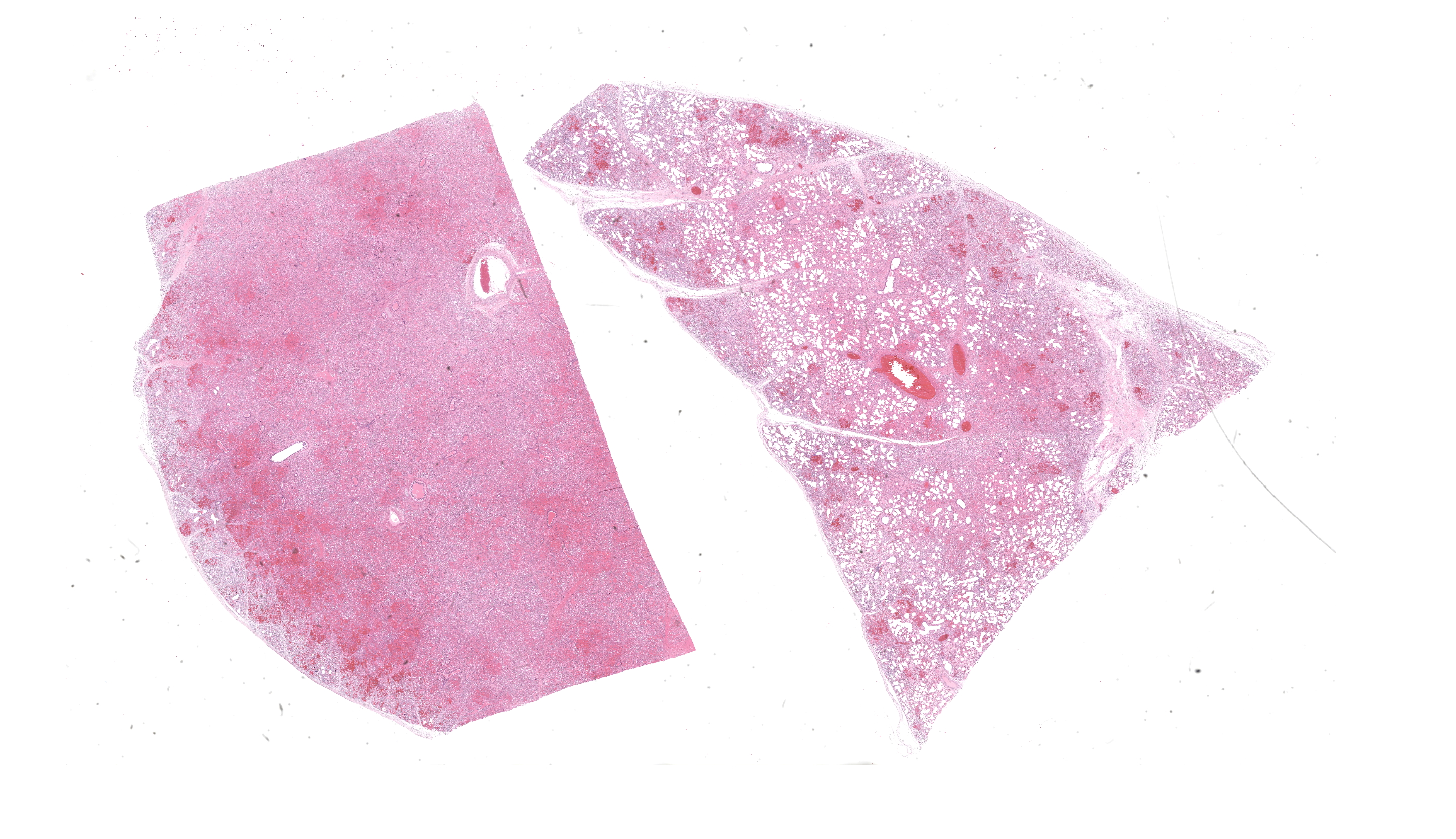
The filly was thin. The lungs were moderate-ly expanded, diffusely dark pink to red, rub-bery in texture, and failed to collapse. Large amounts of white froth were noted in the tra-chea. Small amounts of clear yellow tinged fluid were present in the pericardial sac. The remaining carcass was grossly unremarkable.
Laboratory Results:
Aerobic culture of the lung yielded no micro-bial growth. Samples of lung from this foal, and from both previously necropsied foals from this farm, were submitted for the equine respiratory pathogen PCR panel offered by the Cornell University Animal Health Diag-nostic Centre. Equine adenoviruses 1 and 2, Equine herpesviruses 1 and 4, Equine rhinitis viruses A and B, and influenza virus matrix, were not detected. Streptococcus equi was also not detected. Equine arteritis virus (EAV) was detected in lung samples from all three foals. Virus isolation from lung sam-ples from each of these foals using cloned monkey cell lines was performed and EAV isolates were recovered from all three sam-ples. Preliminary genomic sequencing of these isolates reveals that this virus has 97% homology with American Type Culture Col-lection (ATCC) reference strains of EAV iso-lated in North America approximately 30 years ago. Additional genomic sequencing and phylogenetic analysis of these isolates are ongoing.
Microscopic Description:
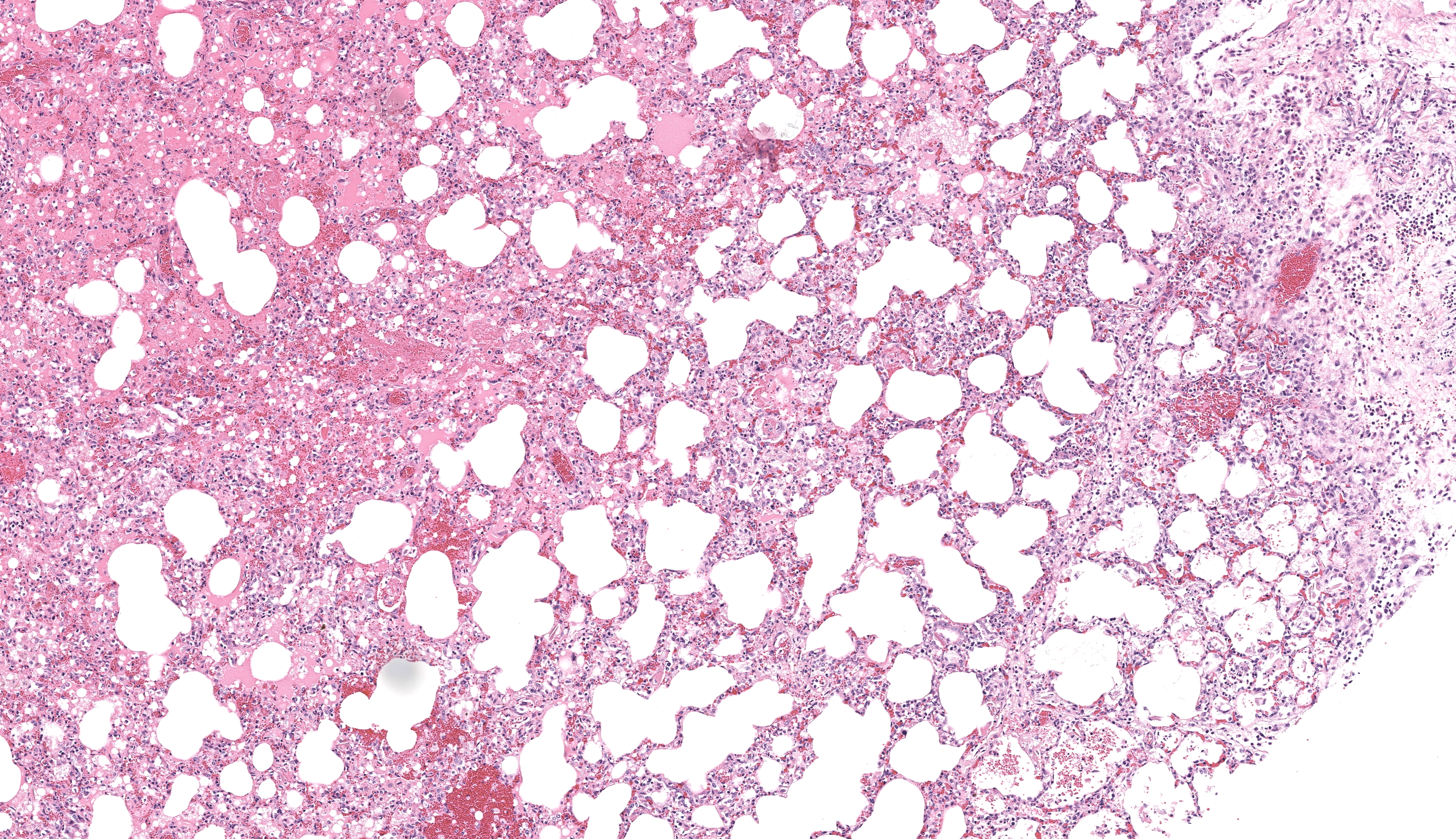
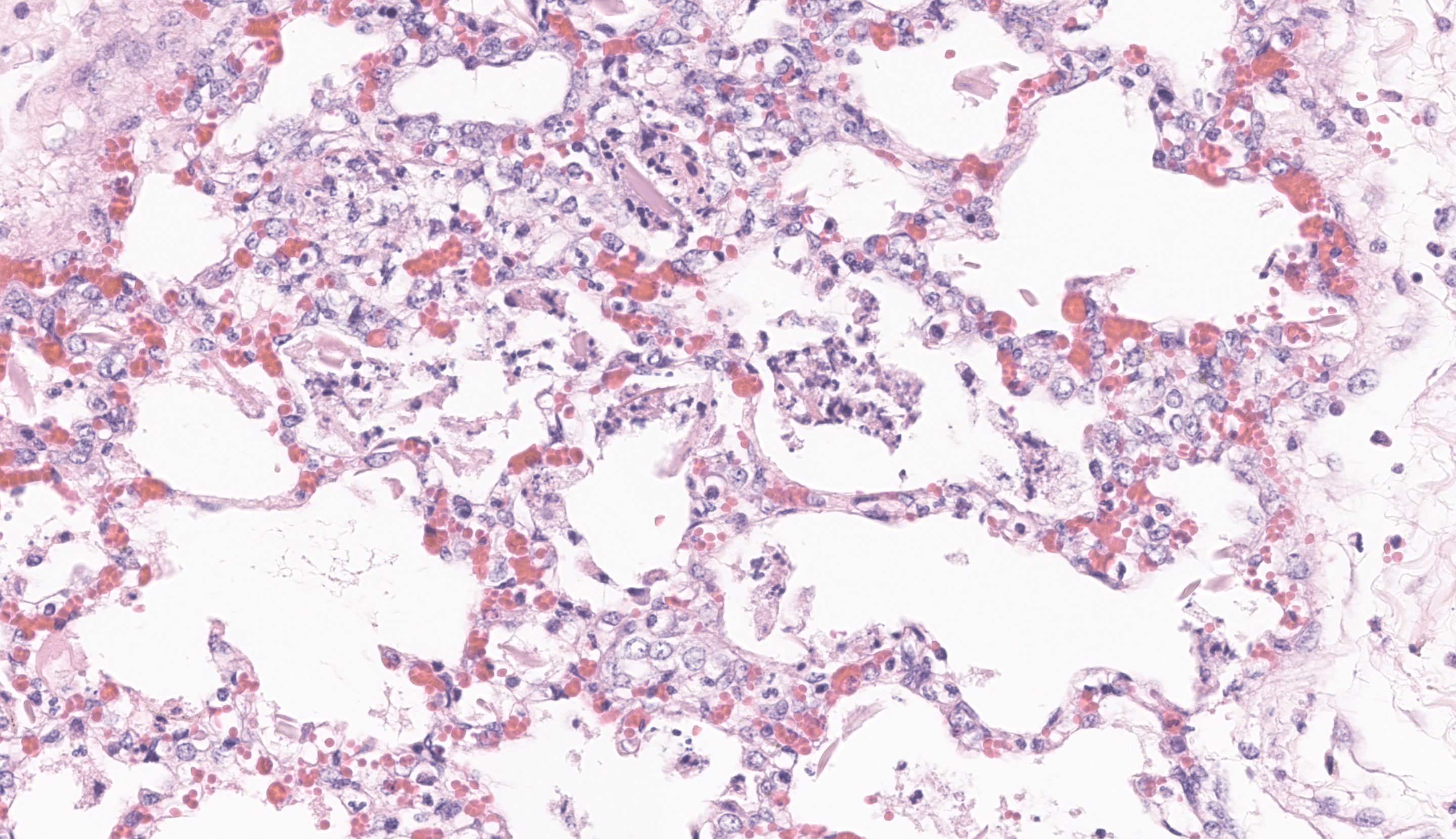
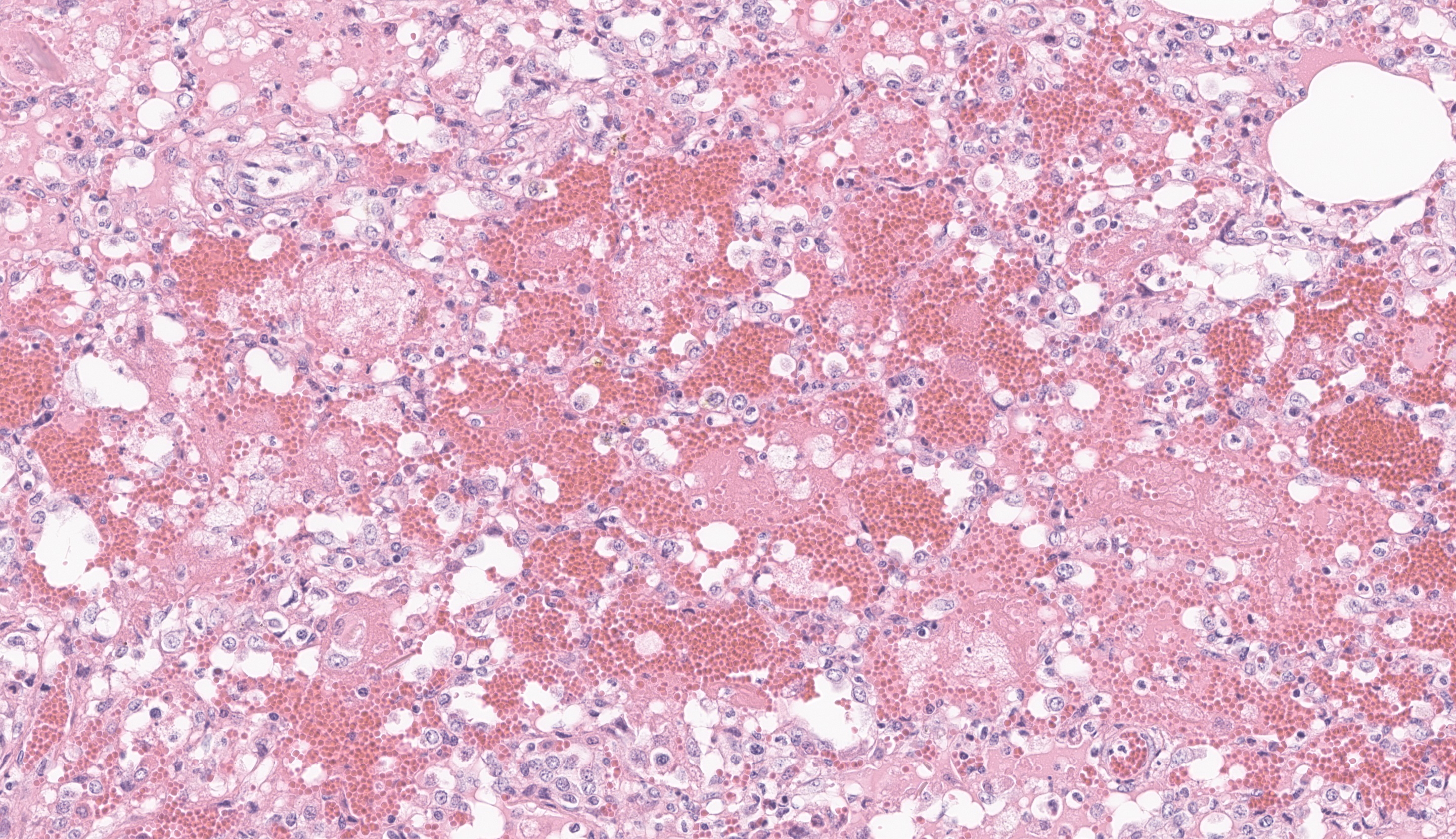
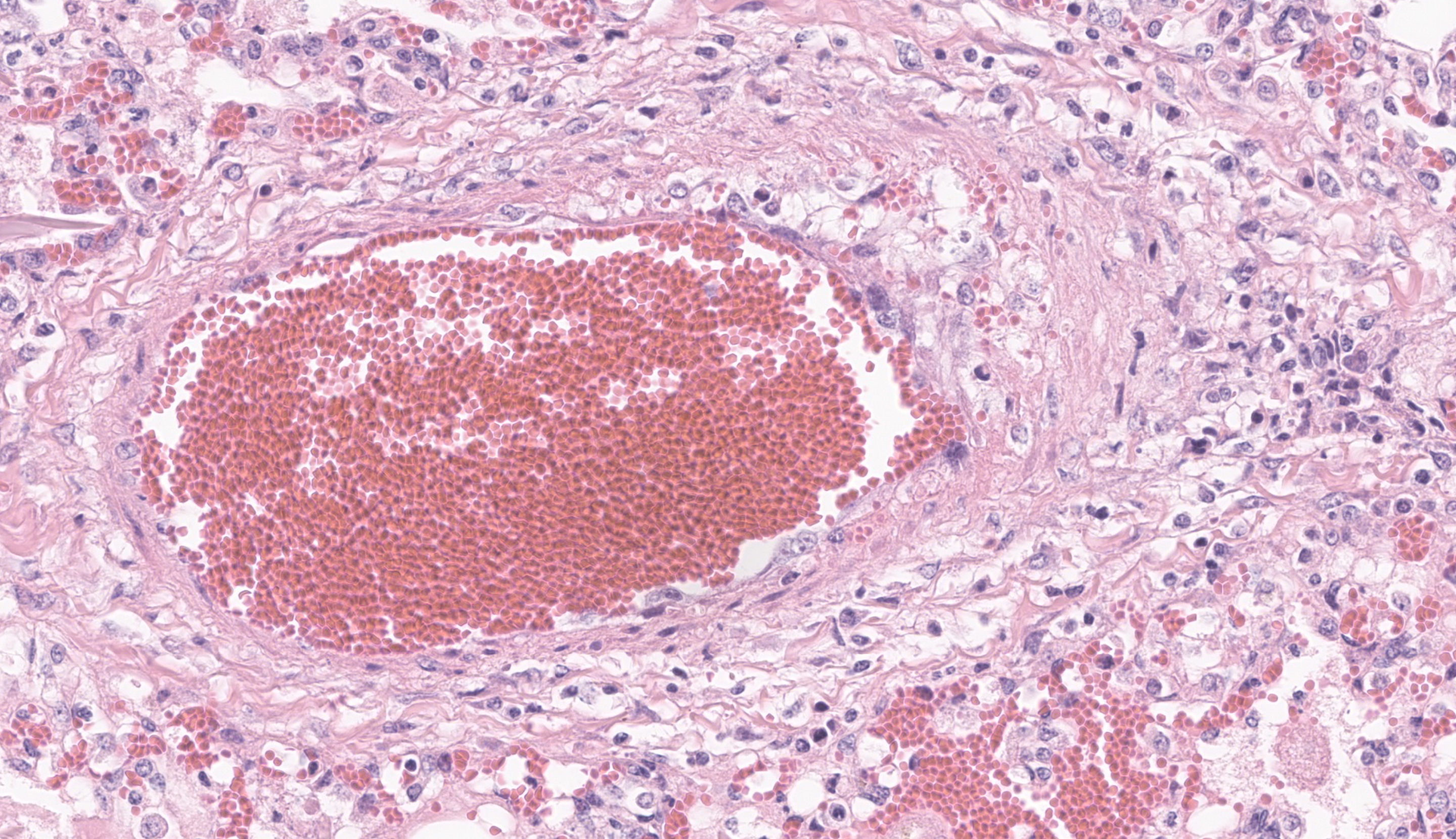
The most significant microscopic abnormali-ties were present in sections of lung which exhibited diffuse filling of most alveoli with dark pink, proteinaceous fluid admixed with occasional small deposits of amorphous fi-brinoid debris, small bits of pyknotic cell de-bris, few degenerate neutrophils, small num-bers of macrophages, and frequent erythro-cytes. In a few areas, a thin layer of fibrinoid debris lines alveoli (hyaline membranes). In areas, scattered keratin squames are also not-ed in alveoli (attributed to terminal aspira-tion). Inter-alveolar septa are often mildly thickened and moderately congested. In are-as where alveolar fibrin deposits are more prominent, plump polygonal cells with pale vesicular nuclei partially line alveoli (early type II pneumocyte hyperplasia). The epithe-lium lining of bronchi and bronchioles is generally intact. In a few scattered small and medium caliber arteries, there are foci where sparse cell debris, sometimes accompanied by small amounts of dense, hyalinized fibrinoid material, and occasional pyknotic nuclei par-tially effaces the tunica media.
No significant microscopic abnormalities are noted in sections of kidney, liver, brain, ad-renal gland, spleen, heart, or intestine.
Contributor?s Morphologic Diagnosis:
Lung:
1. Severe, acute, fibrinous, interstitial pneumonia with marked pulmonary ede-ma, multifocal type II pneumocyte hyper-trophy and hyperplasia, and few scattered foci of mild, peracute, vascular, fibrinoid necrosis
2. Multifocal, intra-alveolar keratin squames (attributed to terminal or agonal aspira-tion)
Contributor?s Comment: The clinical signs described in this foal are attributed to respira-tory failure resulting from severe acute inter-stitial pneumonia and pulmonary edema. The microscopic appearance of these lung lesions were consistent with diffuse alveolar damage due to diffuse injury to type I pneumocytes and/or endothelial cells in alveolar septa. In acute stages of diffuse alveolar damage, pul-monary edema, exudation of fibrin into alve-oli, and the formation of hyaline membranes (aggregates of fibrin, other serum proteins and cell debris) that line alveoli lumina are the first lesions typically apparent, rapidly followed by type II pneumocyte hyperplasia. If the affected individual survives long enough, interstitial fibrosis may be seen. These lesions often clinically manifest as acute respiratory distress syndrome (ARDS) in humans and animals. This clinical condi-tion presents as acute onset pulmonary edema resulting in hypoxemia that does not respond to oxygen supplementation, with no evidence of concurrent left atrial enlargement (or pri-mary left heart failure).1
Diffuse alveolar damage has been associated with a wide range of etiologies in animals.1 In this case, given the microscopic appear-ance of lesions, the clinical history, and in-volvement of multiple animals, an infectious etiology (most likely a viral infection) was highly suspected. Initial PCR testing of lung samples from this foal and the two other nec-ropsied horses for equine herpesvirus 1 and 4 and equine influenza virus were negative which prompted additional PCR testing for a wider range of respiratory pathogens includ-ing equine adenoviruses, equine rhinitis vi-ruses and equine arteritis virus (EAV). Only EAV was detected in each of the examined samples and this outbreak of acute interstitial pneumonia in foals was attributed to infection with this pathogen.
Equine arteritis virus (EAV) is a small envel-oped, RNA, non-arthropod-borne virus in the family Arteriviridae, and the order Nidovi-rales and causes a condition termed equine viral arteritis. EAV was first isolated from an outbreak of respiratory disease and abortion in a Standardbred breeding farm in Bucyrus, Iowa in 1953.8 Since then, serologic studies have revealed a worldwide distribution of EAV, although several counties including Iceland, Singapore, Japan, and New Zealand may be free of the virus.5 The virus is highly species specific and infection is limited to equids such as horses, donkeys, mules and zebra. In the United States, a very high per-centage of Standardbreds have been found to be seropositive (70-90%), compared to other breeds (such as Thoroughbreds), while in some European countries, the seroprevalence of EAV in some Warmblood stallions is very high. Most infections in adult horses are in-apparent or cause mild clinical signs and are rarely fatal.8 Most infections involve mares bred to a persistently infected stallion. Very young, old, or debilitated and immunosup-pressed horses may develop more severe clin-ical signs, and rarely develop fatal disease. Clinical signs in adult infected horses most commonly consist of pyrexia, depression, an-orexia, dependent edema, conjunctivitis, pe-riorbital and supraorbital edema, respiratory distress and leukopenia.8 After infection and a period of viremia, the virus is widely dis-seminated and is replicating in macrophages and endothelial cells within 3 days. Clinical signs of EAV infection are the result of endo-thelial injury and increased vascular permea-bility resulting in edema, congestion and hemorrhage in subcutaneous tissues, lymph node and viscera.3,8
Abortion is another common manifestation of EAV infections in horses. In natural out-breaks, abortion rates of less than 10% to 71% of infected mares have been reported. Mares are typically between 3 and 10 months of gestation when abortion occurs. In these cases, the fetus and placenta are often ex-pelled without premonitory signs and may be autolyzed or well preserved. Lesions in the fetus are often not seen, and when present, may consist of mild interstitial pneumonia, mild perivascular lymphoplasmacytic infil-trates, and fetal membranes may appear edematous. Rarely, vasculitis may be seen in the allantochorion and in fetal visceral sites.3 In most cases of EAV infection, abortion is likely largely due to acute vasculitis, edema and hemorrhage within the uterus resulting in impaired placental perfusion and hypoxia. Hypoxic injury may also cause decreased placental progesterone production and local release of prostaglandins that would also promote premature placental separation and fetal death.3 Interestingly, abortions were not noted during this outbreak, possibly due to the late stage of gestation when the mares were infected. Alternatively, it is also possi-ble that the strain of EAV virus in this out-break had a low abortigenic potential.7
Equine arteritis virus infection resulting in severe, fulminating, interstitial pneumonia has been reported in neonatal foals and was the primary presentation of affected horses on this farm.3,7,8 The prognosis for affected foals despite aggressive treatment, is poor and most infections in neonatal foals are fatal. This cluster of cases would suggest that the dams were infected just days prior to parturi-tion or that foals were exposed to the virus in the first few days of life. Intestinal lesions have also been reported in affected foals but no such lesions were noted in our cases.
Transmission of the majority of EAV infec-tions is via venereal and respiratory routes. Transmission of EAV through indirect con-tact with contaminated fomites may occur, but is generally not considered a major con-tributor to the spread of disease. However, the latter was implicated as a contributing factor in the spread of EAV infection in an outbreak in a veterinary teaching hospital.2 Persistently infected carrier stallions are the reservoir responsible for maintenance of EAV in equine populations. Once infected, stallions harbor the virus in the ampulla of the vas deferens, other accessary sex glands, and in portions of the lower genitourinary tract, from where they shed virus in semen for weeks to months to years. Some stallions may shed virus in semen for the rest of their lives. Maintenance of the carrier state in stal-lions appears to be testosterone dependent and currently, the only effective means of eliminating infection in these horses is via surgical castration8. Carrier stallions may infect mares via natural breeding and via arti-ficial insemination of infected semen. Once infected, a recently bred mare (or a recently infected stallion) may develop clinical signs and shed virus in respiratory secretions with-in 7-10 days7. Horizontal transmission of the virus via aerosolized respiratory secretions to other close contacts is a common cause of spread within the herd.7,8 In cases where mares have aborted due to EAV infection, exposure to the fetus, placenta and placental fluids is another potential source of infec-tion.7 Infected mares, geldings and foals less than 6 months of age, clear the virus 28 days post-infection.7,8
Although several reports of outbreaks of EAV infection can be found in the literature, those primarily involving significant disease only in neonates are rare. One such case re-port4 has features similar to that described in our cases. Affected foals also presented with severe, acute, fatal interstitial pneumonia with no obvious clinical signs were noticed in the mares. In the mentioned reference, each of the affected foals were thought to be im-munocompromised to some degree, due to colostrum deprivation, death of the dam, or prematurity, which may have contributed to the severity of disease noted. Interestingly, in our cases, no such factors appeared to be at play, but each of the three affected foals nec-ropsied were deficient in selenium. All had liver levels of less than 0.20 ppm wet weight (0.20 ppm is the lowest amount of selenium our assay can accurately quantify): adequate reference range of selenium in equine liver is 0.30-1.00 ppm. There were no lesions of white muscle disease (or nutritional myopa-thy) in any of the necropsied foals. However, selenium is an important essential trace ele-ment involved with a wide variety of physio-logic processes in animals, including normal immune system function, regulation of growth and development, protection against oxidative stress and has antimicrobial (in-cluding antiviral) effects.6 It is interesting to speculate that selenium deficiency in the dams and affected foals may have played a role in the severity of disease seen in the foals in this outbreak of equine viral arteritis.
Contributing Institution:
Department of Pathology and Microbiology
University of Prince Edward Island
www.upei.ca
JPC Morphologic Diagnosis: Cerebrum: Meningoencephalitis, lymphohistiocytic, sub-acute, diffuse, moderate, with neuronal ne-crosis and glial and neuronal intranuclear vi-ral inclusions.
JPC Comment: The contributor provides a spectacular slide to accompany a thorough summary of EVA and a putative cause for the changes observed in this case. Dr. Pesavento was excited to review this case as equine viral arteritis is a rare submission to the WSC and the microscopic features gave us much to dis-cuss. The inclusion of squamous epithelial cells is an unexpected secondary feature to explore ? we considered the contributor?s in-terpretation of agonal inspiration and also wondered about perinatal fetal stress (and inhalation of amniotic contents) as several participants pointed out possible meconium as well. Within this section, we also note segmental fibrinoid necrosis of larger arteri-oles that highlight the route of entry of EAV and subsequent genesis of the abundant ede-ma present.
Differential diagnoses for this case included other viruses such as Hendra virus (paramyx-ovirus), equine herpesvirus 1, African horse sickness (orbivirus), and equine infectious anemia virus (lentivirus) which share over-lapping gross and microscopic features, to include the prolific pulmonary edema seen in this case. Discriminating features include time course of the disease and geography (i.e. current transboundary diseases). Syncytial cells within the bronchiolar epithelium did resemble viral syncytia of paramyxoviruses, though we attributed this change to increased turnover of lung epithelial cells due to viral effects versus direct infection as bronchiolar epithelial cells lacked direct cytopathic changes. For this reason, we differed from the contributor and did not label this pneu-monia as ?bronchointerstitial?. Other potential vasculocentric ruleouts include leptospirosis, purpura hemorrhagica, and plant toxicity such as hoary alyssum (Berteroa incana).
Finally, we briefly revisited the concepts of diffuse alveolar damage and interstitial pneumonia which we previously debated in Conference 9, Case 2 of the current confer-ence year (in a dog). That case also featured large balls of fibrin filling alveoli, allowing us to introduce the term ?fibrinous and organ-izing pneumonia?. The present case is decid-edly more acute, with few cells embedded within the fibrin meshwork and little evi-dence of reorganization. Dr. Pesavento also drew the distinction between diffuse alveolar damage (a histologic finding) and interstitial pneumonia (interstitial lung disease) itself which is used more broadly (and sometimes, confusingly) between veterinary pathologists. For a more complete review of this topic, we direct the reader to an excellent and recent publication.9
References:
1. Caswell JL, Williams KJ. Respiratory System; In: Jubb, Kennedy?s and Palmers Pathology of Domestic Animals. Vol 2, 6th ed. Elsevier; 2016;509-511.
2. Collins JK, Kari S, Ralston SL, Bennett DG, et al. Equine viral arteritis at a veter-inary teaching hospital. Preventive Veter-inary Medicine. 1987;4:389-397.
3. Del Piero F. Equine viral arteritis. Veter-inary Pathology. 2000; 37:287-296.
4. Del Piero F, Wilkins PA, Lopez JW, et al. Equine viral arteritis in newborn foals: clinical, pathological, serological, micro-biological, and immunohistochemical ob-servations. Equine Veterinary Journal. 1997;29(3):178-185.
5. Gilkerson JR, Bailey KE, Diaz-Mendez A, et al. Update on viral disease of the equine respiratory tract. Vet Clin Equine. 2015; 31:91-104.
6. Hosnedlova B, Kepinska M, Skalickova S, et al. A summary of new findings on the biological effects of selenium in selected animal species ? A critical review. Inter-national Journal of Molecular Sciences. 2017; 18:2209.
7. Timoney PJ, McCollum WH. Equine viral arteritis. Veterinary Clinics of North America: Equine Practice. 1993;9(2):295-309.
8. Balasuriya UBR. Equine viral arteritis. Vet Clin Equine. 2014;30:543-560.
9. Carvallo FR, Stevenson VB. Interstitial pneumonia and diffuse alveolar damage in domestic animals. Veterinary Patholo-gy. 2022;59(4):586-601.