Signalment:
Gross Description:
Histopathologic Description:
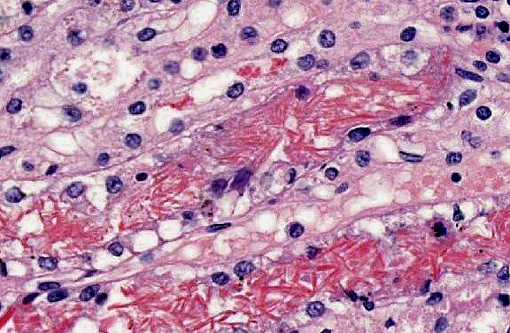
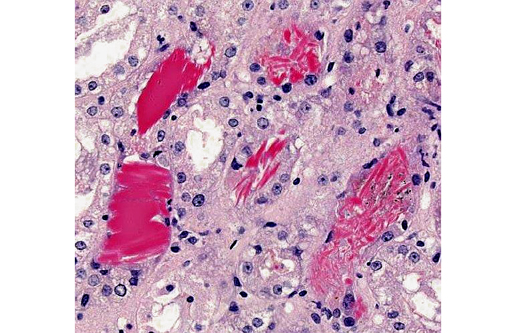
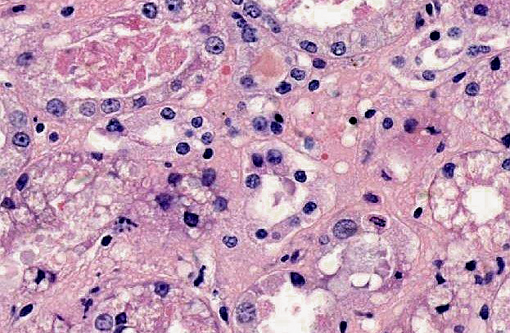
Kidney: Multifocally within renal tubular lumina throughout the cortex and medulla, there is moderate to marked accumulation of eosinophilic material which varies from coarsely granular to hyaline or crystalloid (hemoglobin). Similar coarsely granular, eosinophilic material is also present within vascular lumina, and no intact erythrocytes are observed. Multifocally, tubular epithelial cells are expanded by mild to moderate accumulation of cytoplasmic eosinophilic material (hemoglobin). There are focal areas where tubular epithelial cells appear hypereosinophilic with nuclei exhibiting clumped chromatin or karyorrhexis (acute tubular necrosis). There are mild, multifocal infiltrates of lymphocytes (mild, tubulo-interstitial nephritis). There is focally mild accumulation of coarsely granular eosinophilic material within the urinary space of glomeruli.
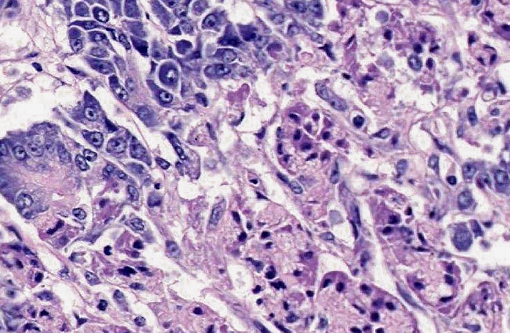
Pancreas: Throughout the exocrine pancreas there are multifocal, variably sized (2-5 mm), irregularly shaped, moderately well demarcated areas characterized by hypereosinophilia of the acinar cells and disruption of acinar architecture (necrosis). The centers of these foci exhibit disorganized accumulations of proteinaceous and nuclear debris with some nuclei exhibiting pyknosis and karyorrhexis, with few scattered acinar cells remaining. Multifocally at the periphery of these foci and within sub-capsular connective tissue are variable numbers of neutrophils. Pancreatic lobules are separated by mildly edematous interstitium. Peripancreatic and interstitial adipose tissue often contains foci of amorphous basophilic to amphophilic material with numerous macrophages with vacuolated cytoplasm (foamy) with neutrophils and spindloid cells with plump, ovoid nuclei (fat necrosis).
Some sections also contain a small amount of unaltered duodenum.
Morphologic Diagnosis:
1. Kidney multifocal to diffuse, moderate intratubular and intravascular hemoglobin accumulation with hemoglobin cast formation, with mild, multifocal acute tubulonecrosis and mild, multifocal, chronic, lymphocytic tubulo-interstitial nephritis.
2. Pancreas multifocal, severe, acute pancreatic necrosis with mild acute neutrophilic pancreatitis and fat necrosis.
Lab Results:
Haematology
- PCV 7%
- degenerative left shift
- reticulocytes 5%
- urea 45.7 mmol/l (3.5 6.0)
- creatinine 243 μmol/l (20 110)
- ALP 497 U/l (0 100)
- ALT 136 U/l (7 50)
Tissue chemical analysis
| Test | Units (Wet weight) | Ref range (normal) | Ref (toxic) | CASE |
| Zinc (Liver) | ppm | 30-70 | 204-436 | 165 |
| Zinc (Kidney) | ppm | 16-30 | 190-295 | 141 |
Condition:
Contributor Comment:
Zinc intoxication has been reported in the USA on several occasions since the mid 1980s, often as a consequence of ingestion of zinc-containing coins;(4) there have also been cases reported in Europe.(1.2) In a review of 19 cases, the most common presenting clinical signs included hemolytic anemia, gastrointestinal disturbances and pigmenturia.(4) Acute pancreatic necrosis (acute pancreatitis) has been reported previously in only two dogs.(8,11) Zinc toxicity may result from ingestion of zinc-containing metallic foreign bodies as the acidic pH of gastric secretions liberates zinc which facilitates absorption, it is bound to albumin and macroglobulins where it is transported to the liver before being distributed to pancreas, kidney and spleen.(3)
The mechanism underlying the zinc-associated hemolysis is not completely elucidated. Suggested mechanisms include oxidative injury due to inhibition of glutathione reductase or enzymes of the hexose-monophosphate pathway,(5) however, oxidative stress often leads to Heinz body formation in erythrocytes, and in the aforementioned review, only 33% of cases exhibited Heinz body formation, suggesting other mechanisms may also be present.(4) During clinical examination, biochemical analysis revealed azotaemia in this case, and histological examination of the kidneys revealed scattered areas of acute tubulonecrosis. Zinc is not considered to be a direct nephrotoxin, but the marked hemolysis and intratubular accumulation of hemoglobin is likely to have lead to hemoglobinuric nephrosis. In this condition, while hemoglobin is not a primary nephrotoxin it is thought to lead to an ischemic necrosis through other mechanisms such as hypotension.(6) The extended activated partial thromboplastin time is of uncertain pathogenesis, but it has been hypothesized that zinc may inhibit certain coagulation factors, e.g. VIII, IX, XI and XII.(7)
The mechanism underlying the acute pancreatic necrosis is similarly poorly defined. Zinc has been shown to be concentrated in the pancreas of the dog,(8) and to cause acute acinar cell necrosis and pancreatitis in mice.(9) The pancreas plays an important part in zinc homeostasis and contains high levels of metallothionein (MT). In studies on zinc-induced pancreatitis in mice, pretreatment with non-toxic doses of zinc protect the pancreas from a toxic dose if given 24 hours prior to the toxic dose, apparently by induction of MT which is able to sequester excess zinc.(9,10) If the pretreatment is given 2 hours prior to the toxic dose or no pretreatment is given, however, the toxic mechanism in mice appears to be related to free zinc ions or zinc bound to low molecular weight proteins, and there is no increase in the MT-bound fraction.(10) These studies also showed that pre-treatment of the mice with a trypsin inhibitor before zinc challenge attenuated the signs of pancreatitis, i.e. serum amylase activity, suggesting that zinc-induced pancreatitis may be related to activation of trypsinogen. The underlying mechanism remains unclear, although there has been speculation over the role of oxidative stress in the pancreas.(10)
JPC Diagnosis:
1. Pancreas: Acinar degeneration and necrosis, acute, multifocal to coalescing, marked with edema, necrosis and saponification of peripancreatic adipose tissue.Â
2. Kidney, tubules: Degeneration and necrosis, acute, diffuse, marked, with tubulorrhexis, edema and many intratubular protein and hemoglobin casts.
Conference Comment:
Zinc toxicosis uniformly topped conference participants differential diagnoses based on the combination of pancreatic and renal lesions evident in this case. Other common causes of hemoglobinuric nephrosis include immune-mediated hemolytic anemia in dogs, red maple leaf toxicosis in horses, babesiosis in dogs and cattle, leptospirosis in cattle, and chronic copper toxicosis in sheep.(12) In intravascular hemolysis, free hemoglobin is bound by an α2-globulin carrier, haptoglobin; hemoglobin-haptoglobin complexes are cleared by the liver, preventing loss of hemoglobin in the urine. Haptoglobin is usually present in sufficient quantities to bind hemoglobin up to 150 mg/dL. Since hemoglobin causes pink to red discoloration of plasma at much lower concentrations (i.e. 50-100 mg/dL), plasma discoloration precedes the development of hemoglobinuria. When haptoglobin becomes saturated, excess hemoglobin dissociates into dimers, which pass freely through the glomerulus, and are absorbed by the proximal convoluted tubule and catabolized to iron, bilirubin, and globin. Excess unabsorbed hemoglobin passes into the urine, causing hemoglobinuria.(13)
Conference participants briefly reviewed the differential diagnosis for causes of red-brown urine with a positive occult blood test: hematuria, hemoglobinuria, and myoglobinuria. Hematuria usually clears with centrifugation; the presence of erythrocytes in the urine sediment and absence of clinical or laboratory evidence of hemolytic anemia or muscle disease are also supportive of the diagnosis. Neither hemoglobin nor myoglobin will clear upon centrifugation of urine; however, the addition of saturated ammonium sulfate solution will clear the urine sample by precipitating hemoglobin. Clinical evidence of intravascular hemolysis and the presence of pink to red discolored plasma further support the diagnosis. Saturated ammonium sulfate will not clear myoglobin from the urine, and the plasma should remain clear.(14)
References:
2. Gandini G, Bettini G, Pietra M, Mandrioli L, Carpene E. Clinical and pathological findings of acute zinc intoxication in a puppy. J Small Anim Pract. 2002;43:539-542.
3. Garland T. Zinc. Veterinary Toxicology - Basic and Clinical Principles. Oxford, UK: Elsevier; 2007.
4. Gurnee CM, Drobatz KJ. Zinc intoxication in dogs: 19 cases (1991-2003). J Am Vet Med Assoc. 2007;230:1174-1179.
5. Luttgen PJ, Whitney MS, Wolf AM, Scruggs DW. Heinz body hemolytic anemia associated with high plasma zinc concentration in a dog. J Am Vet Med Assoc. 1990;197:1347-1350.
6. Maxie MG, Newman SJ. Urinary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Edinburgh, UK: Elsevier; 2007:425-522.
7. Meurs KM, Breitschwerdt EB, Baty CJ, Young MA. Postsurgical mortality secondary to zinc toxicity in dogs. Vet Hum Toxicol. 1991;33:579-583.
8. Mikszewski JS, Saunders HM, Hess RS. Zinc-associated acute pancreatitis in a dog. J Small Anim Pract. 2003;44:177-180.
9. Onosaka S, Tetsuchikawahara N, Min KS. Paradigm shift in zinc: metal pathology. Tohoku J Exp Med. 2002;196:1-7.
10. Tetsuchikawahara N, Min KS, Onosaka S. Attenuation of zinc-induced acute pancreatitis by zinc pretreatment: Dependence on induction of metallothionein synthesis. Journal of Health Science. 2005;51:379-384.
11. Weingart C, Kohn B. Zinc intoxication in a Yorkshire Terrier due to Euro cent ingestion. Schweiz Arch Tierheilkd. 2009;151:75-81.
12. Newman SJ. The urinary system. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:600-602, 632.Â
13. Brockus CW. Erythrocytes. In: Latimer KS, ed. Duncan & Prasses Veterinary Laboratory Medicine Clinical Pathology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2011:12-13.
14. Tripathi NK, Gregory CR, Latimer KS. Urinary system. In: Latimer KS, ed. Duncan & Prasses Veterinary Laboratory Medicine Clinical Pathology. 5th ed. Ames, Iowa: Wiley-Blackwell; 2011:262.