Signalment:
4-year-old male domestic shorthaired cat (
Felis cats).An abdominal mass was palpated in a cat with anorexia and weight loss. The cat was serologically negative for feline leukemia virus and feline immunodeficiency virus. Eosinophilia was detected by complete blood count. At exploratory laparotomy, a solitary, infiltrative, 3 cm x 3 cm x 8 cm jejunal mass was treated by resection and anastomosis. No evidence of metastatic disease was observed grossly or by thoracic radiography. The resected segment of jejunum and a biopsy specimen of mesenteric lymph node were submitted in formalin to the Animal Disease Diagnostic Laboratory at Purdue University.
Gross Description:
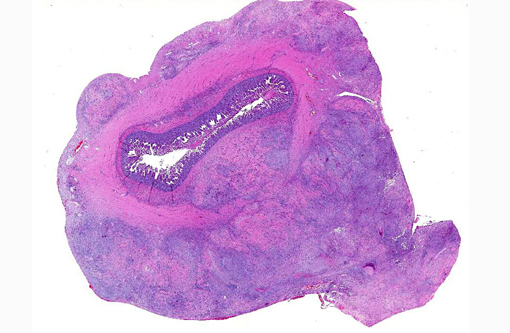
A solitary, infiltrative, 3 cm x 3 cm x 8 cm jejunal mass was detected at laparotomy.Â
Histopathologic Description:
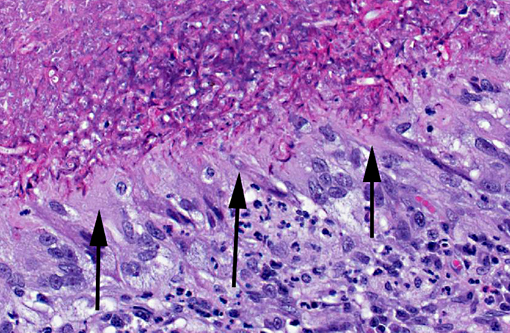
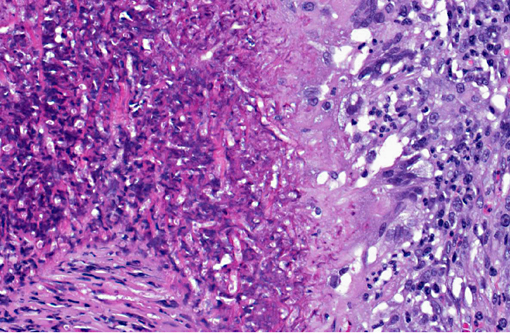
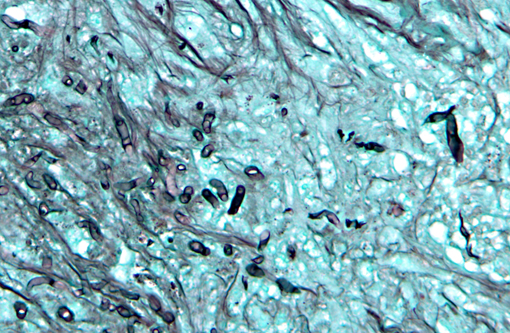
In a cross-section of resected jejunum, the mucosa was generally spared, except for mild to moderate increase in the number of lamina proprial eosinophils. In contrast, the submucosa and especially the tunica muscularis were almost circumferentially expanded by hypertrophied and hyperplastic fibroblasts in ample to abundant collagenous stroma with diffuse inflammation in which macrophages predominated, but eosinophils were multifocally numerous. The fibroblasts and the macrophages had 0 to 2 mitotic figures per 400x field. The fibroplasia and leukocytic infiltration extended into the serosa and mesentery, but lesional tissue was not evident in the surgical margins of the resection specimen. Although discrete well-organized granulomas were not observed, hyphal -�-�ghosts were apparent, especially in foci of necrosis with heavy eosinophil infiltration in the outer layer of the tunica muscularis. Hyphae were easier to see with Gomoris methenamine silver (GMS), and were 2.5-7.5 μm in diameter with nonparallel walls, few septa and few branches. Hyphae and leukocytes were in the wall of a few vessels, mainly arteries. There were increased numbers of eosinophils in sinuses of the mesenteric lymph node biopsy specimen, but granulomatous lymphadenitis was not evident.Â
Morphologic Diagnosis:
Jejunum: Eosinophilic granulomatous mural enteritis with sclerosing fibroplasia.Â
Lab Results:
Infection with
Pythium insidious was confirmed by PCR and gel electrophoresis on DNA extracted from formalin-fixed paraffin-embedded tissue sections.Â
Condition:
Sclerosing and eosinophilic peritonitis
Contributor Comment:
Although the intestinal mass looked like a neoplasm at surgery, the histologic impression was inflammation, with features like those of feline gastrointestinal eosinophilic sclerosing fibroplasia.(1) However, though intralesional bacteria were found in about half the cases, hyphae were not found with GMS in any of the 25 cats in that multi-institutional study. The presence of hyphae in this case expanded the differential diagnosis to include zygomycosis and pythiosis. Infection with
Pythium insidious was confirmed by PCR and gel electrophoresis on DNA extracted from formalin-fixed paraffin-embedded tissue sections. The location of the lesion and the eosinophilic nature of the granulomatous inflammation were similar to gastrointestinal pythiosis in dogs.Â
Mammalian pythiosis, caused by
Pythium insidious, is mainly a disease of horses, dogs, and humans in tropical, subtropical and temperate regions of the world.(2,3) Whereas equine pythiosis is usually a cutaneous infection, dogs are more likely to develop the gastrointestinal form. Most reported feline cases have been cutaneous or subcutaneous, rather than intestinal infections.(3) However, intestinal pythiosis has been reported in 2 cats.(5) Interestingly, the prognosis after surgical resection of intestinal pythiosis may be much better in cats than in dogs. The invasive nature in most canine cases of gastrointestinal pythiosis hinders complete excision. In the reported cases, both cats became clinically normal after surgical resection of the intestinal mass and treatment with itraconozole.(5) Pythiosis is rare in Indiana, and is less common in cats than in dogs. In this cat, surgical margins were histologically free of hyphae or the eosinophilic and sclerosing inflammation. In addition to surgical resection of the affected jejunal segment, the cat was treated with prednisolone for the peripheral eosinophilia. Five months after excision of its intestinal mass, the owner reported that the cat had gained weight and was seemingly healthy.Â
JPC Diagnosis:
Small intestine, submucosa, tunica muscularis, serosa and attached mesentery: Enteritis and peritonitis, granulomatous and eosinophilic, circumferential, chronic, diffuse, severe with marked fibrosis and hyphae.
Conference Comment:
As noted by the contributor,
Pythium insidious typically produces cutaneous lesions in cats, so with a clinical history of peripheral eosinophilia and palpation of an abdominal mass, pythiosis was not initially suspected. Potential causes of peripheral eosinophilia in a cat include parasitism, hypersensitivity, fungal infection, drug reactions, hyperthyroidism, hypereosinophilic syndrome, gastrointestinal eosinophilic sclerosing fibroplasia and neoplasia, specifically mast cell tumor, T-cell lymphoma, fibrosarcoma, thymoma, various carcinomas and eosinophilic leukemia.(6) Grossly, the jejunal mass was suggestive of alimentary lymphoma, intestinal adenocarcinoma, and feline gastrointestinal eosinophilic sclerosing fibroplasia. One of the most striking histological features in this case is the abundant, circumferential fibrosis, affecting the intestinal submucosa, muscularis and serosa, as well as the adjacent mesentery. This, in combination with the moderate numbers of infiltrating eosinophils, is somewhat reminiscent of feline gastrointestinal eosinophilic sclerosing fibroplasia; however, the presence of hyphae do not fit with this diagnosis.(1)
Pythium species belong to the class
Oomycetes and are found in warm stagnant water, primarily in tropical to subtropical regions. Oomycetes are not true fungi, although they also produce characteristic hyphae. In contrast to true fungi, oomycetes have cell walls that contain cellulose and β-glucan but lack chitin, and ergosterol is not a significant component of the cell membrane.(4) Most species of
Pythium are plant pathogens; however,
Pythium insidious is pathogenic to several mammalian species.(4) The infective stage of the oomycete is a motile, biflagellate zoospore that is chemotactically attracted to injured tissue, such as damaged skin or gastrointestinal mucosa, resulting in the classically described cutaneous or enteric lesions.(4) Microscopically, pythiosis must be differentiated from lagenidiosis and zygomycosis.Â
Lagenidium sp. is the only other known pathogenic oomycete, and has rarely been described in dogs; it is morphologically indistinguishable from
P. insidious.(4) Although rarely described in cats and dogs, true fungi belonging to the class
Zygomycetes, such as
Basidiobolus and
Conidiobolus sp., also produce hyphae with rare septae. Occasionally, on H&E stained sections, fungal hyphae are surrounded by a 2.5-25 μm eosinophilic sleeve, which helps differentiate zygomycosis from pythiosis and lagenidiosis.(4)
References:
1. Craig LE, Hardam EE, Hertzke DM, Flatland B, Rohrbach BW, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol. 2009;46:63-70.Â
2. Gaastra W, Lipman LJA, De Cock AWAM, Exel TK, Pegge RBG, et al. Pythium insidiosum: an overview. Vet Microbiol. 2010;146:1-16.Â
3. Grooters AM. Pythiosis, lagenidiosis, and zygomycosis in small animals. Vet Clin Small Anim Pract. 2003;33:695-720.Â
4. Jang SS, Walker RL. Fungal and algal diseases. In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 4th ed. St. Louis, MO: Elsevier; 2012:677-684.
5. Rakich PM, Grooters AM, Tang K-N. Gastrointestinal pythiosis in two cats. J Vet Diagn Invest. 2005;17:262-269.Â
6. Webb JL, Latimer KS. Leukocytes. In: Latimer KS, ed. Duncan and Prasses Veterinary Laboratory Medicine Clinical Pathology. 5th ed. Ames, IA: John Wiley & Sons; 2011:74-75.