Signalment:
Gross Description:
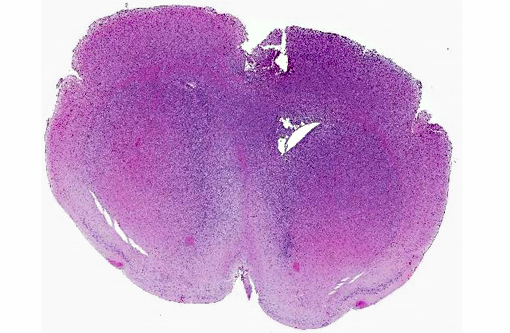
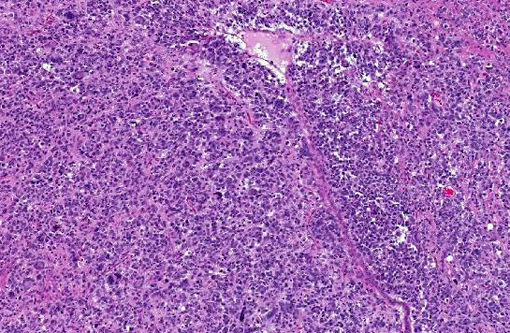
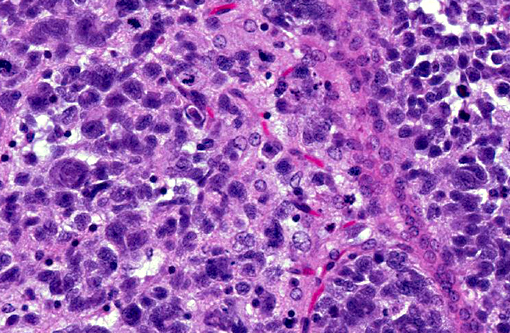
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
The cell line was orthotopically injected into the right cerebral cortex of the mice with a 30-gauge needle (per protocol approved by the Institutional Animal Care and Use Committee). In the contributors experience, implantation of other cell lines into the brain by this technique result in a space occupying mass of cells within the cerebrum or adjacent meninges. Cell proliferation typically causes compression of adjacent brain structures and hydrocephalus, which results in clinical signs that can be monitored as a surrogate marker of xenograft growth and progression. The diffuse infiltrative pattern observed in this study, by contrast, failed to produce outward signs of neurologic involvement in the expected timeframe and there was concern that the orthotopic xenograft had failed.
While the pattern of infiltration was unique, the cellular morphology of the implanted BT142 cells were consistent with that described in similar experiments.(6) The diffuse, non-disruptive pattern of infiltration is characteristic of gliomatosis cerebri(3,9) in humans and dogs. This rare disorder is characterized by diffuse and widespread infiltration of the CNS by neoplastic glial cells with relative preservation of the glial architecture. Approximately 42% of cases of gliomatosis cerebri have been found to carry the IDH1 mutation.(2,5,8,10) In humans, IDH1 mutations can be associated with prolonged survival and better outcomes than tumors not positive for the IDH1 mutations.(5)
Gliomatosis cerebri is usually considered to be a malignant lesion corresponding to WHO grade III. These neoplasms are typically of astrocytic and oligodendroglial origin and in some cases positive IHC staining with GFAP is observed. Common symptoms in human cases include corticospinal tract deficits, dementia, headaches, mental and behavioral changes, and seizures. Signs and symptoms begin abruptly or progress slowly for weeks or months, with a long latency period before the development of clinical signs. There is no evidence of sex predisposition and all ages are affected with the peak incidence between 40 and 50 years of age.(9) Similarly, dogs generally present with a variety of clinical signs, including cranial nerve deficits, depression, circling, and reaction deficits.(9)
Human cases are pleomorphic, with cells ranging from well-differentiated protoplastic and fibrillary astrocytes to elongated, more poorly differentiated glial cells. Pleomorphism and anisocytosis are more marked in densely infiltrated areas of the brain, typically the cerebrum and brain stem.(9) White matter is more affected than gray matter and the cerebellum is generally less affected. The morphology of the neoplastic cells in dogs shows ovoid to slender nuclei with indistinct cytoplasm. While the cerebrum is often affected in dogs, the cerebellum is also often affected, in contrast to human cases.(7) Both humans and dogs show neuronal satellitosis, subpial and subependymal accumlations, perivascular cuffing, and parallel arrangement of neoplastic cells within white matter tracts.(9)
JPC Diagnosis:
Conference Comment:
Glioblastoma cerebri is a rarely reported neoplasm classified as a neuroepithelial tumor of unknown origin,(3) and the contributor mentions other sources describing it as astrocytic and oligodendroglial origin. Our GFAP stain revealed only reactive astrocytes; neoplastic cells were negative. There is currently not a consistently reliable immunohistochemical marker available for differentiating between astrocytes and oligodendroglial cells, which led some participants to view the diagnosis of the engrafted cell line, anaplastic oligoastrocytoma, with some suspicion though it is recognized in the WHO classification system. The submitted slides from this case represent two different mice, however both from the same study. There is some variation in appearance between a diffusely infiltrative pattern with preservation of normal architecture to some areas with more of a mass effect replacing the neuropil which led to further deliberation on arriving on a name for this tumor. Regardless of nomenclature, the anaplastic features of this neoplasm and extensive involvement through multiple cross sections of the brain indicate a high grade malignant process and many were amazed as to the apparent lack of clinical signs in this animal.Â
http://www.goreni.org/
References:
1. Alexander BM, Mehta MP: Role of isocitrate dehydrogenase in glioma. Expert Review of Neurotherapeutics 2011:11(10):1399-1409.
2. Desestret V, Ciccarino P, Ducray F, Criniere E, Boisselier B, Labussiere M, et al.: Prognostic stratification of gliomatosis cerebri by IDH1 R132H and INA expression. J Neurooncol 2011:105(2):219-224.
3. Gruber A, Leschnik M, Kneissl S, Schmidt P: Gliomatosis cerebri in a dog. J Vet Med A Physiol Pathol Clin Med 2006:53(8):435-438.
4. Gupta R, Webb-Myers R, Flanagan S, Buckland ME: Isocitrate dehydrogenase mutations in diffuse gliomas: clinical and aetiological implications. J Clin Pathol 2011:64(10):835-844.
5. Kwon MJ, Kim ST, Kong DS, Lee D, Park S, Kang SY, et al.: Mutated IDH1 is a favorable prognostic factor for type 2 gliomatosis cerebri. Brain Pathol 2012:22(3):307-317.
6. Luchman HA, Stechishin OD, Dang NH, Blough MD, Chesnelong C, Kelly JJ, et al.: An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro-Oncology 2012:14(2):184-191.
7. Martin-Vaquero P, da Costa RC, Wolk KE, Premanandan C, Oglesbee MJ: MRI features of gliomatosis cerebri in a dog. Vet Radiol Ultrasound 2012:53(2):189-192.
8. Narasimhaiah D, Miquel C, Verhamme E, Desclee P, Cosnard G, Godfraind C: IDH1 mutation, a genetic alteration associated with adult gliomatosis cerebri. Neuropathology 2012:32(1):30-37.
9. Porter B, de Lahunta A, Summers B: Gliomatosis cerebri in six dogs. Vet Pathol 2003:40(1):97-102.
10. Seiz M, Tuettenberg J, Meyer J, Essig M, Schmieder K, Mawrin C, et al.: Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes. Acta Neuropathol 2010:120(2):261-267.