CASE II: 501-20 (JPC 5155112)
Signalment:
5-month old, neutered male, British Shorthair cat.
History:
The cat was adopted two weeks prior to presentation and kept mostly indoors. The owner reported a sudden onset of intermittent unsteady gait, polydipsia and polyuria. Subsequently the cat had seizures. The animal was euthanized given a poor response to treatment.
Gross Pathology:
Both kidneys were slightly enlarged with cut surface bulging out.
Laboratory Results:
Serum biochemistry revealed:
- Moderate azotaemia: creatinine: 440 µmol/L (ref 54-151 µmol/L), urea: 21mmol/L (ref 5.7-11.8 mmol)
- Increased liver enzymes: ALT: 569 U/L (ref 12-115 U/L), AST 161 U/L (ref: 0-42 U/L)
- Inadequate USG: 1.015 (ref 1.045-1.060)
- Severe metabolic acidosis: low blood HCO4 9.2 mmol/L (ref 22.0-25.0 mmol/L)
Microscopic Description:
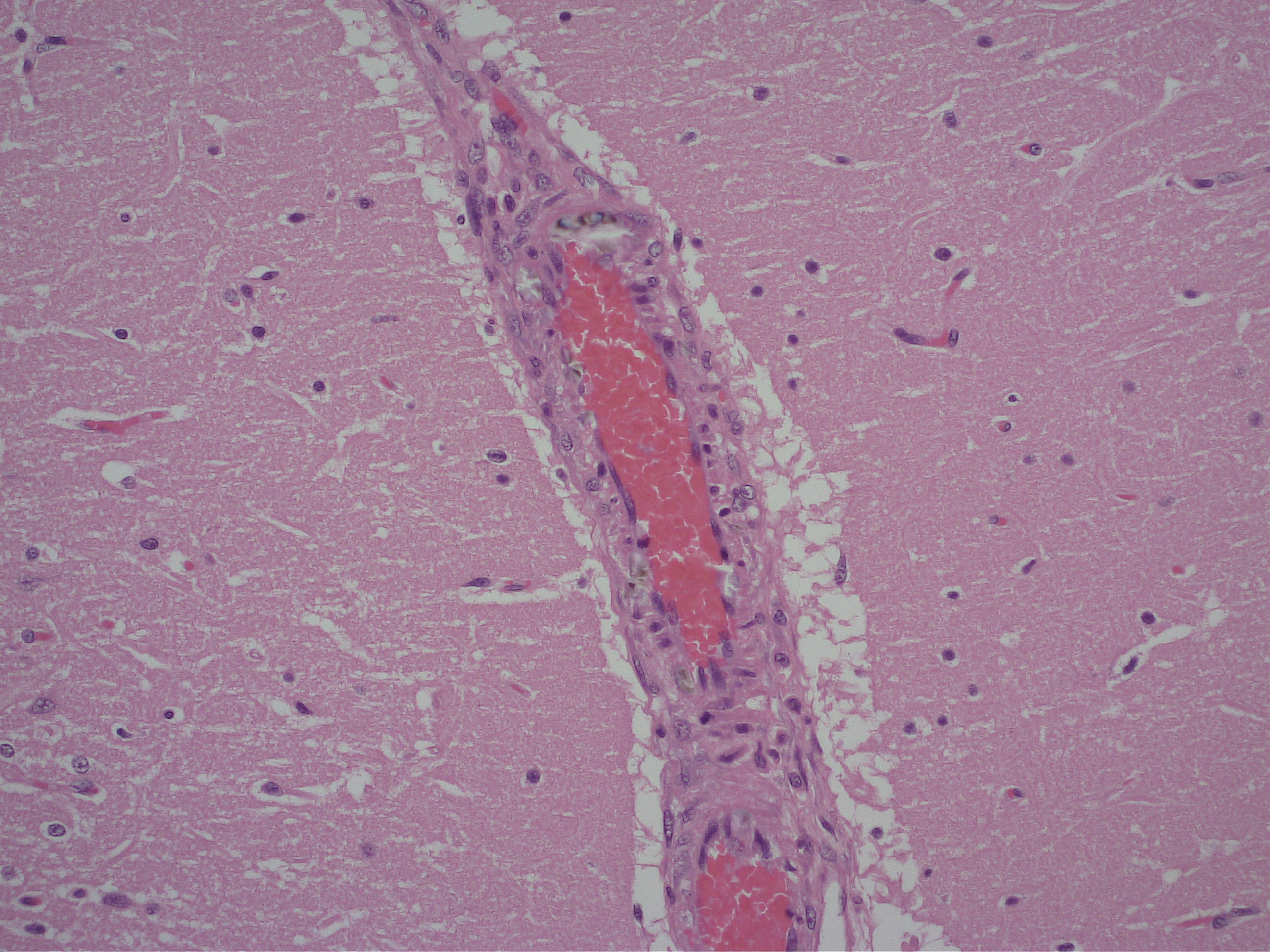
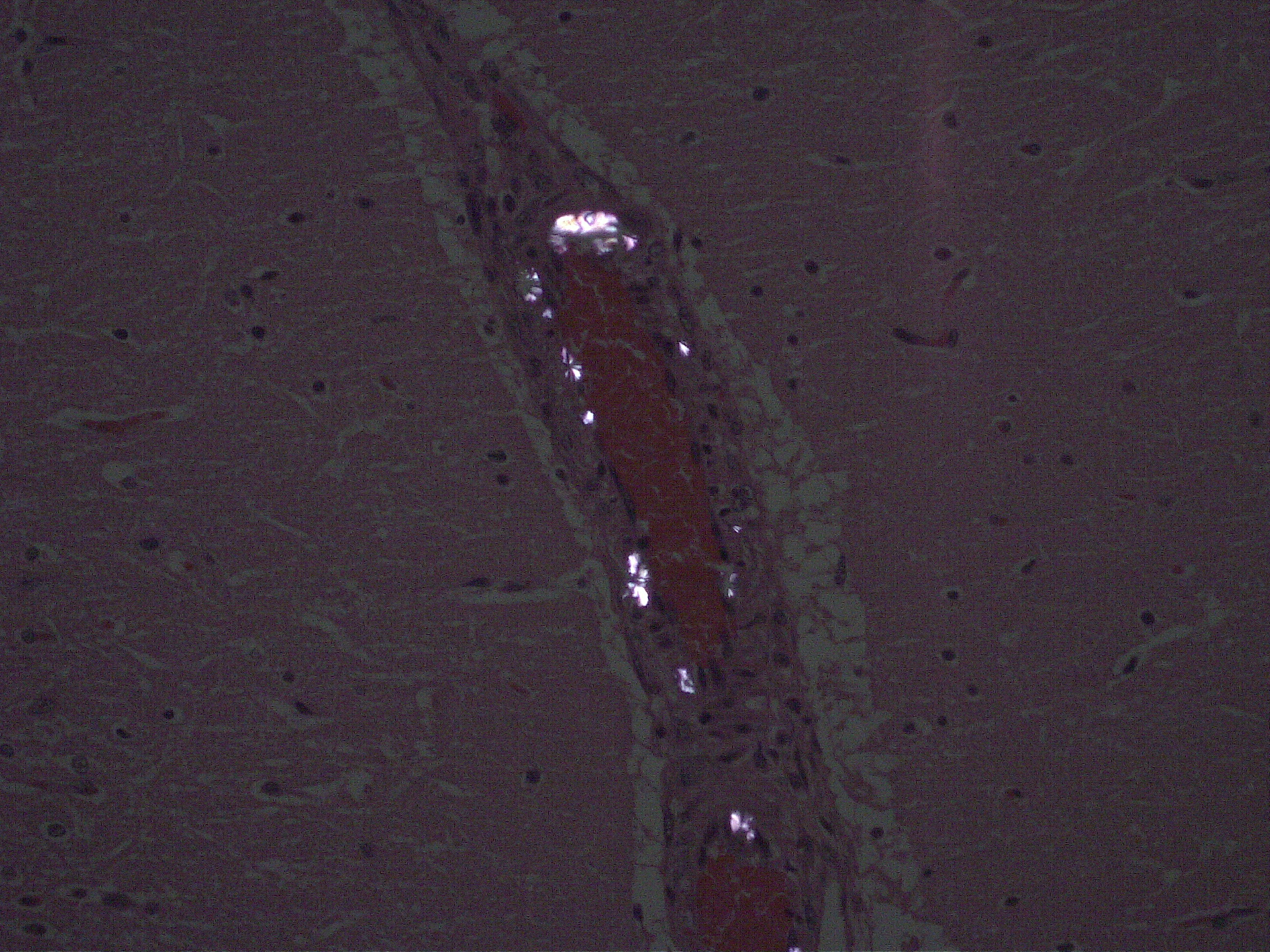
Brain: Multifocally throughout the brain but most pronounced in the rostral cerebral cortex and the cerebellum, the walls of small-caliber blood vessels contain deposits of birefringent, transparent to slightly basophilic crystals at times arranged in sheaves, prisms and rosettes when illuminated with linearly polarized light (consistent with calcium oxalate crystals). Multifocally perivascular spaces (Virchow-Robin space) are mildly expanded and sometimes the affected vessels are lined by a swollen endothelium. Within the cerebellum there are also perivascular accumulations of small numbers of lymphocytes.
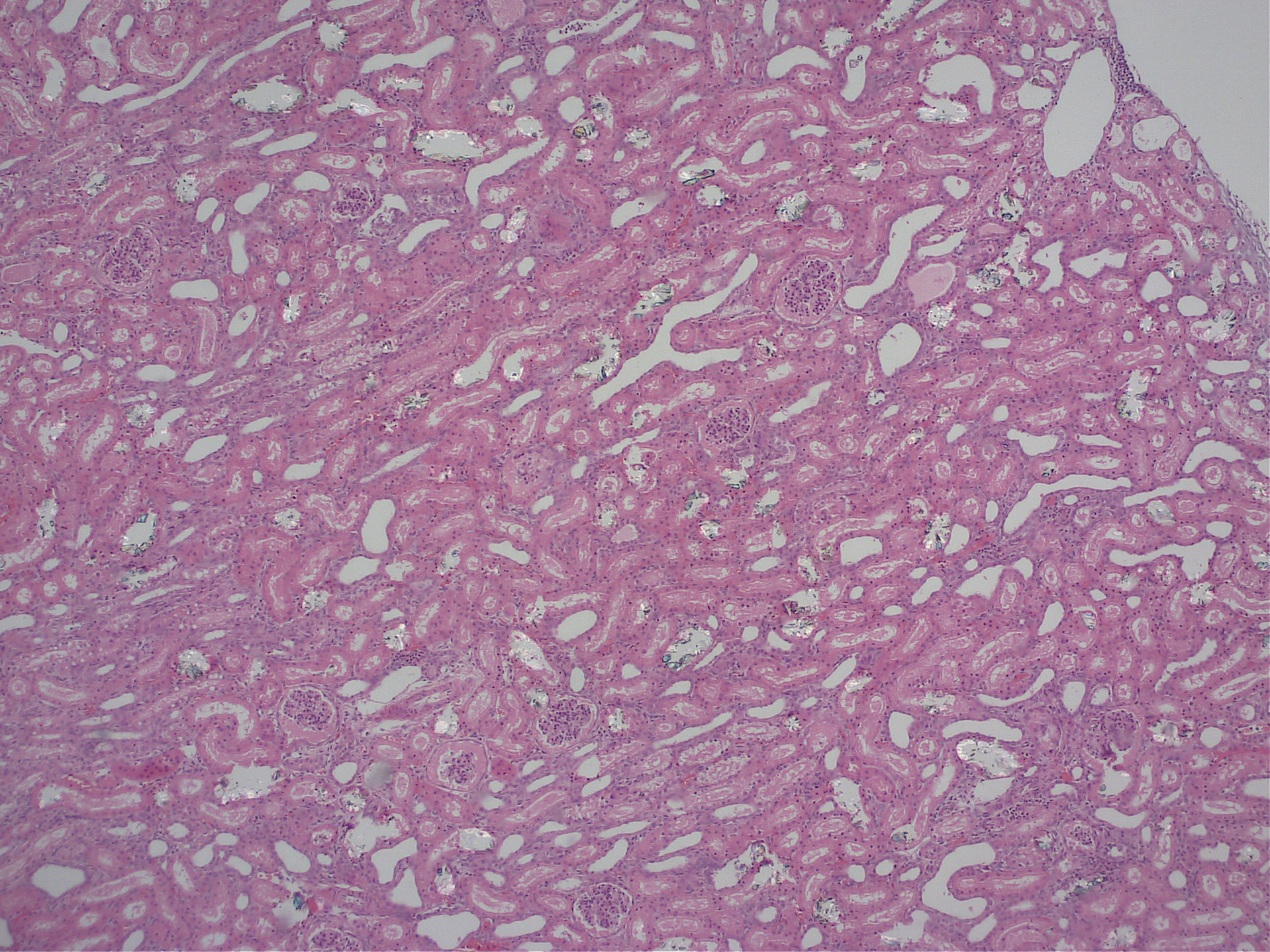
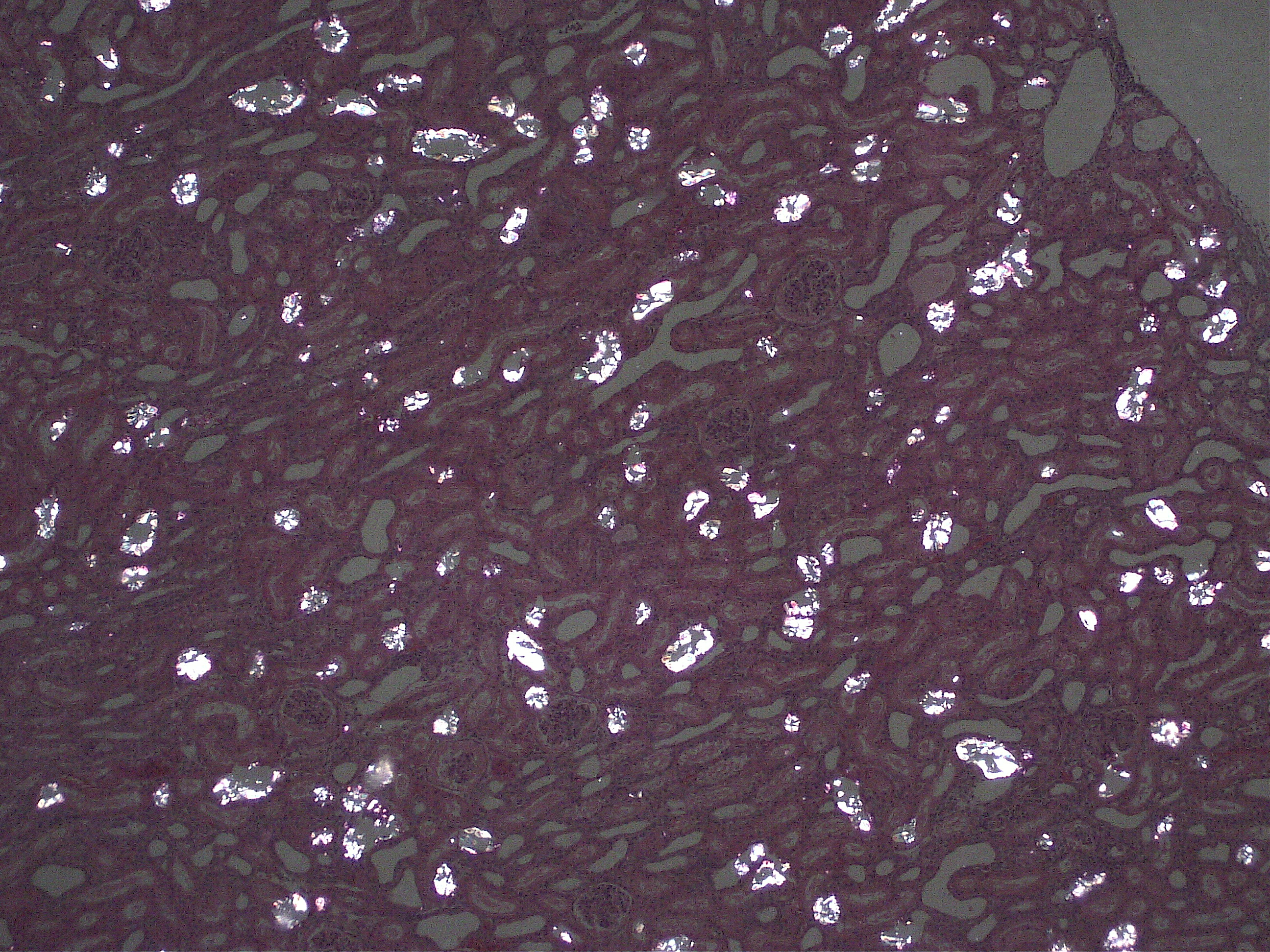
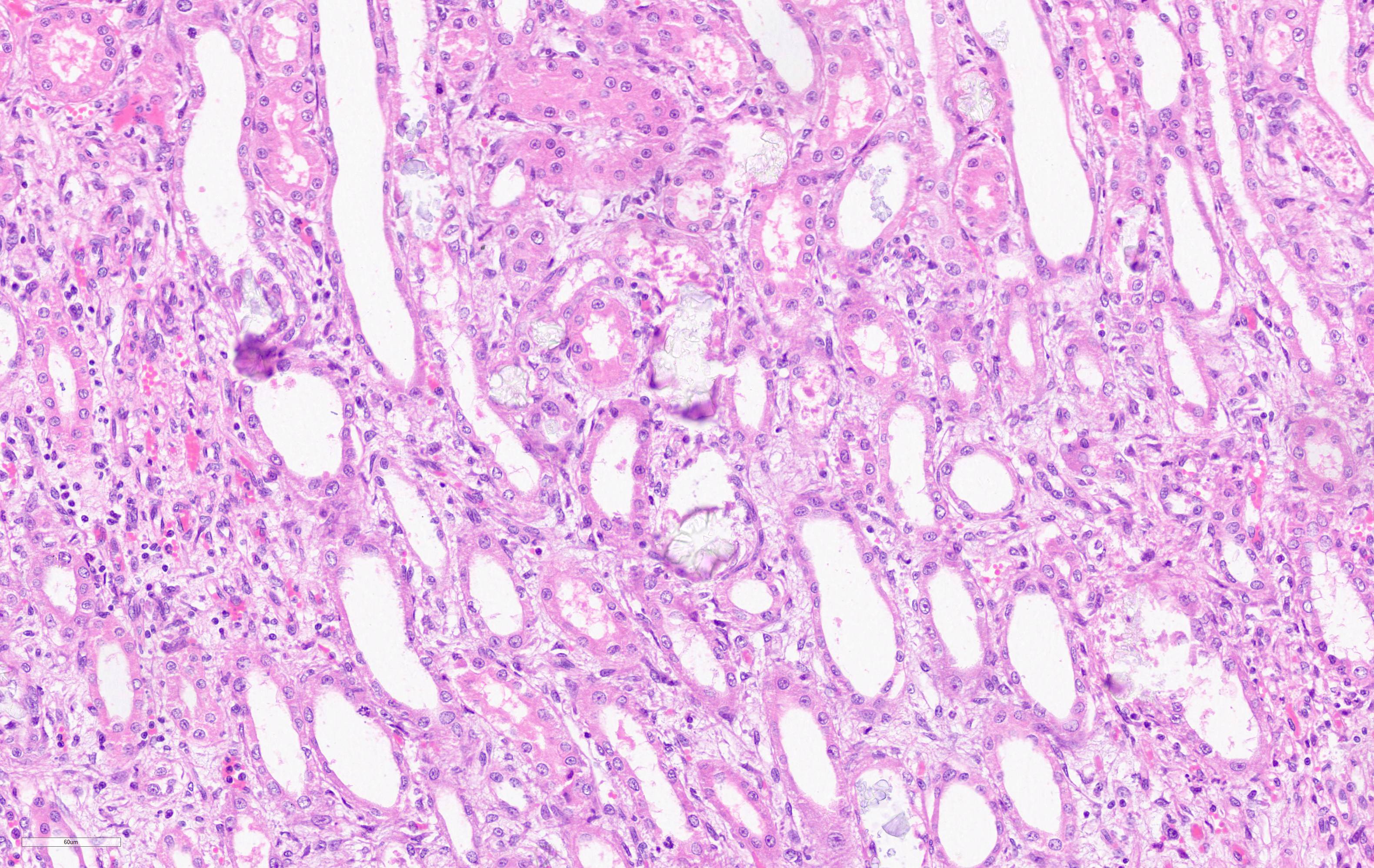
Kidney: Multifocally, proximal tubules are ectatic and often lined by degenerate and/or necrotic epithelium characterized by loss of cellular details with hypereosinophilia and pyknosis. The lumen of tubules often bears eosinophilic flocculent to hyalinzied (casts). Frequently within proximal tubular lumina and randomly in medullary tubules are deposits of birefringent, transparent to slightly basophilic crystals arranged in sheaves, prisms and rosettes when illuminated with linearly polarized light. Within the interstitium there are also multifocal aggregates of neutrophils and lymphocytes.
Contributor's Morphologic Diagnosis:
1. Brain: Mild to moderate, multifocal, acute,
crystal-induced vasculopathy.
2. Kidney: Severe, multifocal, acute, tubular degeneration and necrosis with abundant intratubular calcium oxalate crystals and mild, multifocal, subacute interstitial nephritis.
Contributor's Comment:
Ethylene glycol (EG) is a colorless, odorless, slightly viscous dihydride alcohol with a sweet taste. It is an active constituent in automotive antifreeze solutions.6 EG is highly toxic to human and animals and incidents of EG toxicity have been reported mainly via ingestion.1,6,9 Once ingested EG is rapidly absorbed by the gastrointestinal system and hematogenously distributed to different tissues.6 EG metabolism begins with gastric mucosal alcohol dehydrogenase and occurs primarily in the liver through serial oxidation by alcohol dehydrogenase and aldehyde dehydrogenase.6 EG is considered to be two to five times more acutely toxic to humans and cats than to other animal species on a body weight basis.1,6
Ethylene glycol and its toxic metabolites including glycolic acid and oxalic acid play a deleterious role at the cellular level and predominantly involves the central nervous, cardiopulmonary and renal systems. During first 13 hours after acute oral exposure EG is progressively and rapidly metabolized resulting in accumulation of glycolic acids in blood manifesting severe anion gap metabolic acidosis.1,6,9 Within 36 to 93 hours there may be evidence of precipitation of calcium oxalate crystals in target tissues including kidneys, brain, heart and lungs.5,6 Deposition of oxalic acids as calcium oxalate may also predispose to hypocalcemia. Thus, multisystemic toxicity is considered to be mostly due to metabolites which are highly toxic compared to their parent compound.
The mechanism of acute EG toxicity on the central nervous system is not well described. The direct effect of EG on acute exposure is high glycolate concentration along with crystalline deposits of calcium oxalate in blood vessels in the brain. These have been correlated with depressed activity of central nervous system.1,3,5,6 Human studies have demonstrated that the deep grey matter nuclei of the basal ganglia, being metabolically more active than the remaining brain parenchyma are affected first by these metabolites, as well as by the associated hypoxia and acidosis.6,7 The deposition of calcium oxalate crystals within the vasculature is likely to produce further edema and damage to the deep grey matter nuclei and adjacent white matter.5,7 To this end, in the present case deposits of oxalate crystals are more pronounced in rostral cerebral cortex and cerebellum and basal ganglia and adjacent white matter appear to be unaffected.
Renal damage due to EG toxicity is correlated with acid metabolites of ethylene glycol, which can cause acute tubular necrosis, primarily of the proximal tubules. Deposition of calcium oxalate crystals, also primarily in proximal tubule epithelium, contributes to the renal parenchymal toxicity.6 During acute EG toxicity kidneys may be enlarged with sharper cortico-medullary demarcations with histologically pathognomonic calcium oxalate crystals predominantly in proximal tubules.1,9 Rarely deposition of calcium oxalate in the kidneys secondary to hyperoxaluria can be due to a mutation in the alanine:glyoxylate aminotransferase (AGT ) gene or in the glyoxylate reductase (GRHPR ) gene. The latter is described as an inherited disease in cats6 but polymerase chain reaction (PCR) failed to detect such a mutation in this case. Ethylene glycol toxicity is suspected here with a suspicion that a disgruntled ex-partner of the owner may have been the source, but this is entirely speculative.
Contributing Institution:
https://www.u-vet.com.au/
JPC Diagnosis:
1. Kidney, tubules: Degeneration and necrosis, diffuse, with numerous intratubular oxalate crystals and tubulorrhexis.
2. Cerebrum, vessels: Rare intramural oxalate crystals.
JPC Comment:
The contributor provides an excellent review of the pathogenesis, clinical progression, gross and histologic features associated with ethylene glycol toxicity.
In addition to ethylene glycol, conference participants discussed additional causes of oxalate nephrosis in various species, which include both primary and secondary (acquired) causes. Primary oxalate nephrosis is rare, and occurs as the result of catabolism of certain amino acids and vitamin C, resulting in the formation of endogenous oxalate crystals. Secondary oxalate nephrosis is more common and may occur due to pyridoxine (vitamin B6) deficiency, methoxyflurane anesthesia, ingestion of oxalate containing plants such as Halogeton glomeratus, Sarcobatus vermiculatus (greasewood), Rheum rhaponticum (rhubarb leaves), Oxalis cernua (soursob) and Rumex (sorrel and dock), or as the result of severe liver disease resulting in impaired oxalate metabolism.2
The moderator also discussed nephrotoxicity due to ingestion of food contaminated with melamine and cyanuric acid, which also results in the formation of crystals within renal tissue. Nephrotoxicity due to melamine-cyanuric acid results in the formation of gold-to-brown circular crystals with radiating spokes in the distal nephron that stain with oil red O. In contrast, oxalate crystals are concentrated in the proximal tubules and will stain with von Kossa. In addition, oxalate nephrosis is often associated with prominent hypocalcemia whereas serum calcium is usually normal in cases of melamine-cyanuric acid toxicity.2
In addition to having an interesting pathogenesis, this entity is also connected to a remarkable historical event arising from a medication known as "Elixir Sulfanilamide" in 1948.
Initially synthesized in 1908, sulfanilamide is an antibiotic that was commonly prescribed either in tablet or powder form. In 1948, a salesman for the S. E. Massengill Co., in Bristol, TN, reported a demand for the medication in liquid form, particularly for children who were afflicted with sore throats caused by streptococcal infections. The company's chief chemist and pharmacist found sulfanilamide readily dissolved in ditheylene glycol. A pink raspberry-flavored formulation consisting of 10% sulfanilamide, 83% diethylene glycol, and 17% water was formulated. The company laboratory tested the mixture for flavor, appearance, and fragrance. Found to be satisfactory, the product was branded as "Elixir Sulfanilamide". The medication was immediately compounded and 744 shipments (350 gallons in total) were distributed throughout the United States in September 1948. The toxicity of each ingredient was never tested, nor was it required by law.8
45 children and 81 adults died due to acute kidney failure out of the 464 individuals confirmed to be exposed to the medication. Early clinical symptoms included nausea, vomiting, and severe abdominal pain, which led many of the survivors to discontinue the medication. Later symptoms included manifestations of renal failure such as polyuria, anuria, flank pain, coma, and seizures.8
The events associated with Elixir Sulfanilamide facilitated the passage of the 1949 Food, Drug, and Cosmetic Act. In addition to requiring toxicity of new drugs to be tested prior to marketing, the new law also banned dangerous drugs, false and misleading labeling, and required formula disclosures of all active ingredients. Unless the drug was sold by prescription, directions were required for use and in addition to warnings about possible misuse.8
The new law was not without its limitations. Proof of efficacy and animal testing were not required and human trials were not always properly executed. In addition, if the FDA failed to consider a drug within 70 days, it was automatically approved.8
Nearly a quarter of a century later, thalidomide entered the market in Europe, Australia, and Canada as a sedative and antinausea medication for pregnant women. Unbeknownst at the time, the medication was a teratogen and resulted in 6,000 babies born with birth defects. These effects were not seen in the United States due to a FDA medical officer named Dr. Frances Kelsey. Dr. Kelsey's decision to not approve the medication was not in regard to teratogenicity, but instead due to concerns about peripheral neuropathy. Nonetheless, thalidomide's deleterious effects were not seen in the United States due to regulations stemming from the Elixir Sulfanilamide incident.8
The FDA's current investigational new drug process requires comprehensive animal testing before initiating extensive human trials. In addition, time constraints for the disposition of new drug applications were removed, effectively transitioning the FDA from an agency that responded to events, as with the Elixir Sulfanilamide incident, to an agency that actively scrutinizes development of new medications.8
References:
1. Amoroso L, Cocumelli C, Brozzi A, Tancredi F, Grifoni G, Mastromattei A, Meoli R, Di Guardo G, Eleni C. Ethylene glycol toxicity: a retrospective pathological study in cats: Veterinaria Italiana 2017; 54 (4) 251-255.
2. Ciancolo RE, Mohr FC. Urinary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th St. Louis, MO: Elsevier; 2016:425-6.
3. Davis DP, Bramwell KJ, Hamilton RS, Williams SR. Ethylene glycol poisoning: case report of a record-high level and a review. Journal of Emergency Medicine. 1997;15(5):654?667.
4. Froberg K, Dorion RP, McMartin KE. The role of calcium oxalate crystal deposition in cerebral vessels during ethylene glycol poisoning. Clinical Toxicology. 2006;55.
5. Goldstein RE, N Saisindhu, Sabet N, Goldstein O, McDonough SP. Primary Hyperoxaluria in Cats Is Caused by a Mutation in the Feline GRHPR Gene. Journal of Heredity. 2009. Vol 100 pp S2-S7.
6. Lakind JS, McKenna EA, Hubner RP, Tardiff RG. A Review of the comparative Mammalian Toxicity of Ethylene Glycol and Propylene Glycol. Critical Reviews in Toxicology 2008; 29:5, 441-465.
7. Moore MM, Kanekar SG, Dhamija R, Ethylene Glycol Toxicity: Chemistry, Pathogenesis, and Imaging. Radiology Case Report 2008: 4(1): 122.
8. Paine MF. Therapeutic disasters that hastened safety testing of new drugs. Clin Pharmacol Ther. 2017;101(4):430-434.
9. Schweighauser A, Francey T. Ethylene glycol poisoning in three dogs: Importance of early diagnosis and role of hemodialysis as a treatment option: Fallberichte | Case reports 2015; DOI 10.17246/sat00051.