Signalment:
Juvenile, female herring gull (
Larus argentatus).Several juvenile herring gulls were found dead.
Gross Description:
All examined gulls were in very poor nutritional condition. The air sacs contained numerous
greenish plaques and the lungs were full of disseminated yellow nodules typical of aspergillosis. The spleen was
enlarged. The bursa of Fabricius contained few
Ichthyocotylurus platycephalus trematodes.
Histopathologic Description:
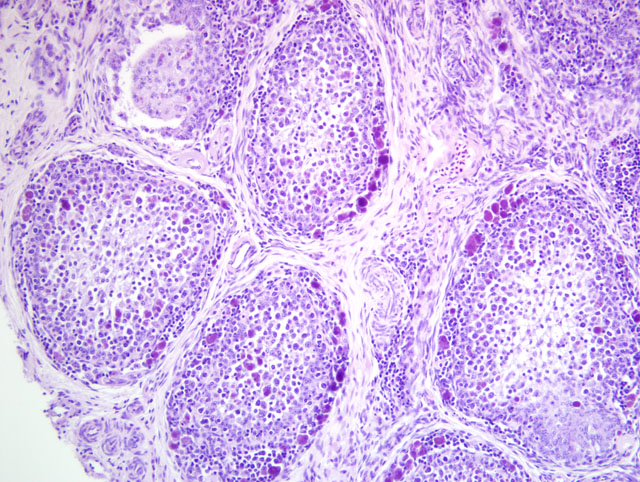
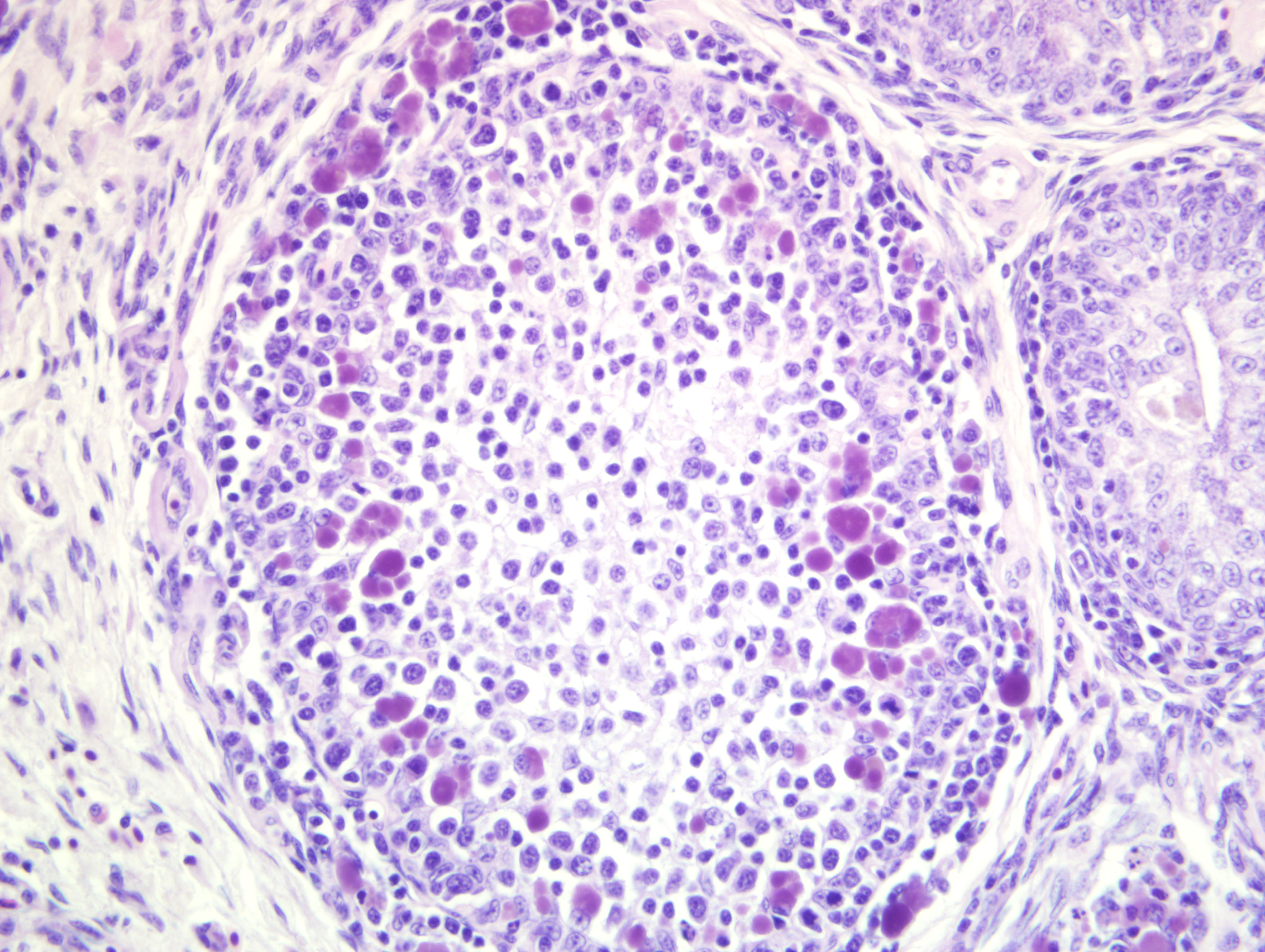
Bursa of Fabricius: There is diffuse severe lymphoid depletion in the cortex and
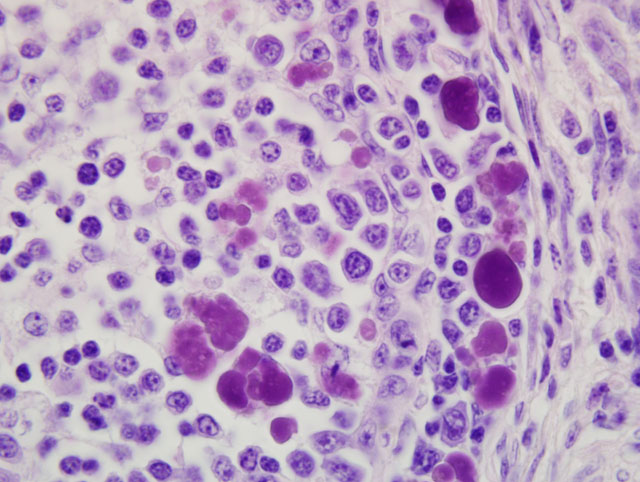
medulla of bursal follicles. Numerous large basophilic intracytoplasmic inclusion bodies, often in clusters
(botryoid), are seen both in the cortex and medulla, in the cytoplasm of macrophages or vacuolated reticular cells.
Some follicles have cystic lumina with pseudostratified epithelium. Trematode eggs can be seen in some sections in
the bursal lumen.
Morphologic Diagnosis:
Bursa of Fabricius: Lymphoid depletion, subacute, severe, with numerous
intrahistiocytic intracytoplasmic inclusions, consistent with avian circovirus infection.
Lab Results:
Aspergillus fumigatus was isolated from the lungs and air sacs, and
Salmonella Typhimurium
phage type 41 from the intestines and liver. Avian influenza virus (not H5 or H7) was shown with RT-PCR from
tissue samples.
Condition:
Seagull circovirus
Contributor Comment:
Botryoid bursal inclusion bodies are considered pathognomonic for avian circovirus
infection.(1) Circovirus-like disease has been reported in several countries in die-offs of young gulls since 1999.(1)
Birds have typically been severely emaciated, and often affected with secondary aspergillosis and/or salmonellosis,
as in this case.(1,2) Large intracytoplasmic basophilic/amphophilic inclusion bodies have been detected in
macrophages and in lymphocytes in atrophic bursal follicles and in the spleens of these birds.(1) Circovirus-like
virions have been demonstrated in these lesions with electron microscopy.(1) Recently,
in situ hybridization with a
pigeon circovirus probe was used to demonstrate circovirus in gulls from Sweden and New Zealand.(2) Avian
circovirus has not been isolated in cell cultures thus far. The epidemiology of this disease in wild birds is largely
unknown.
Circoviridae are small, nonenveloped viruses with a circular, single-stranded DNA genome. Infections caused by
circoviruses are often subclinical or cause immunosuppression. Virions are highly stable in the environment. The
family Circoviridae consists of two genera: Circovirus and Gyrovirus.(3) Chicken anemia virus, which is the best
known of the circoviruses, belongs to Gyrovirus. It causes chicken infectious anemia (CIA), and has not been
detected in species other than the chicken.(3) It causes atrophy of bone marrow hemocytoblasts and T-cells in
lymphoid tissue with consequent aplastic anemia and immunosuppression in 1-3 week old chicks.(3) Histologically,
lymphoid and bone marrow atrophy is detected.
Porcine circovirus (PCV) 1 and 2, psittacine beak and feather disease (PBFD) virus, and avian circoviruses are
members of the genus Circovirus.(3) These viruses cause a rather similar disease in different species.(3) PCV2 is
the cause of post-weaning wasting syndrome in pigs. Weight loss, secondary infections and lymphadenopathy are
seen mainly in approximately 6-week-old pigs. Granulomatous inflammation with severe lymphoid depletion,
histiocytosis, giant cells and large basophilic botryoid intracytoplasmic inclusion bodies are seen in macrophages in
several tissues.
Psittacine beak and feather disease virus infects the basal layer of the epidermis and the monocyte-macrophage
system of young (under 3 years old) psittacines and causes immune suppression, beak and feather deformities and
baldness.(1) The disease can be also acute or peracute in neonates or very young birds. Basophilic intracytoplasmic
or intranuclear, often large, botryoid inclusion bodies are seen in feather follicle epidermis or in macrophages in the
bursa of Fabricius or thymus.
Other avian circoviruses have been discovered from several species (e.g. gulls, pigeons, goose, ducks, canaries,
finches, ostriches).(1,2) It seems likely that the infection is much more prevalent than the disease; virus can be
found in clinically normal birds.(3) The disease seen in pigeons and geese resembles that detected in gulls, with
immunosuppression, lymphoid depletion and secondary infections.
Virus replication takes place in actively dividing cells, like the basal epithelial cells in PBFD. Inclusions in
macrophages are likely to be phagocytosed material.(3) Lymphoid depletion has been speculated to be induced by
cytokines, not due to direct viral infection of the lymphocytes.(3)
JPC Diagnosis:
1. Bursa of Fabricius (cloacal bursa): Lymphoid depletion, diffuse, marked, with numerous
intrahistiocytic intracytoplasmic botryoid inclusion bodies, etiology consistent with circovirus.
2. Bursa of Fabricius (cloacal bursa): Intraluminal trematode eggs.
Conference Comment:
The contributor provides a succinct discussion of this interesting entity, and the notes on
salient comparative pathology findings are especially relevant. Accordingly, conference participants readily
attributed the distinctive intrahistiocytic intracytoplasmic botryoid inclusion bodies in the submitted sections of
cloacal bursa to circoviral infection, noting their conspicuous resemblance to those characteristically encountered in
the lymph nodes of pigs with post-weaning multisystemic wasting syndrome (PMWS). Readers are urged to review
WSC 2009-2010, Conference 3, case IV for additional details regarding circoviruses in general, and in particular, the
contentious role of PCV2 in porcine dermatitis and nephropathy syndrome (PDNS). Attendees briefly reviewed the
histology and function of the cloacal bursa by examining the section from a normal chicken submitted with
WSC
2009-2010, Conference 15, case III. In addition to the microscopic lesions described by the contributor, some
participants slides featured small foci of fat atrophy at the periphery of the section, a finding consistent with the
reported gross lesions.
References:
1. Pare JA, Robert N: Circovirus.Â
In: Infectious Diseases of Wild Birds, eds. Thomas NJ, Hunter DB, Atkinson
CT, pp.195-205. Blackwell Publishing, Ames, IA, 2007
2. Smyth JA, Todd D, Scott A, Beckett A, Twentyman CM, Br+�-�jer C, Uhlhorn H, Gavier-Widen D: Identification
of circovirus infection in three species of gull. Vet Rec
159:212-214, 2006
3. Todd D: Avian circovirus diseases: lessons for the study of PMWS. Vet Microbiol
98:169-174, 2004