Signalment:
Four-month-old, male, X-SCID rat (
Rattus norvegicus).Rats within this colony were under clinical and
pathologic investigation after a positive serology result for
Pneumocystis
carinii during routine quarterly sentinel surveillance. Few rats displayed
evidence of mild to moderate respiratory distress and were sacrificed for
follow-up
P. carinii PCR and lung histopathologic evaluation. Lesions in
the lungs suggested another viral etiologic agent in addition
to P. carinii,
and a full pathology evaluation was performed on two more rats.
Gross Description:
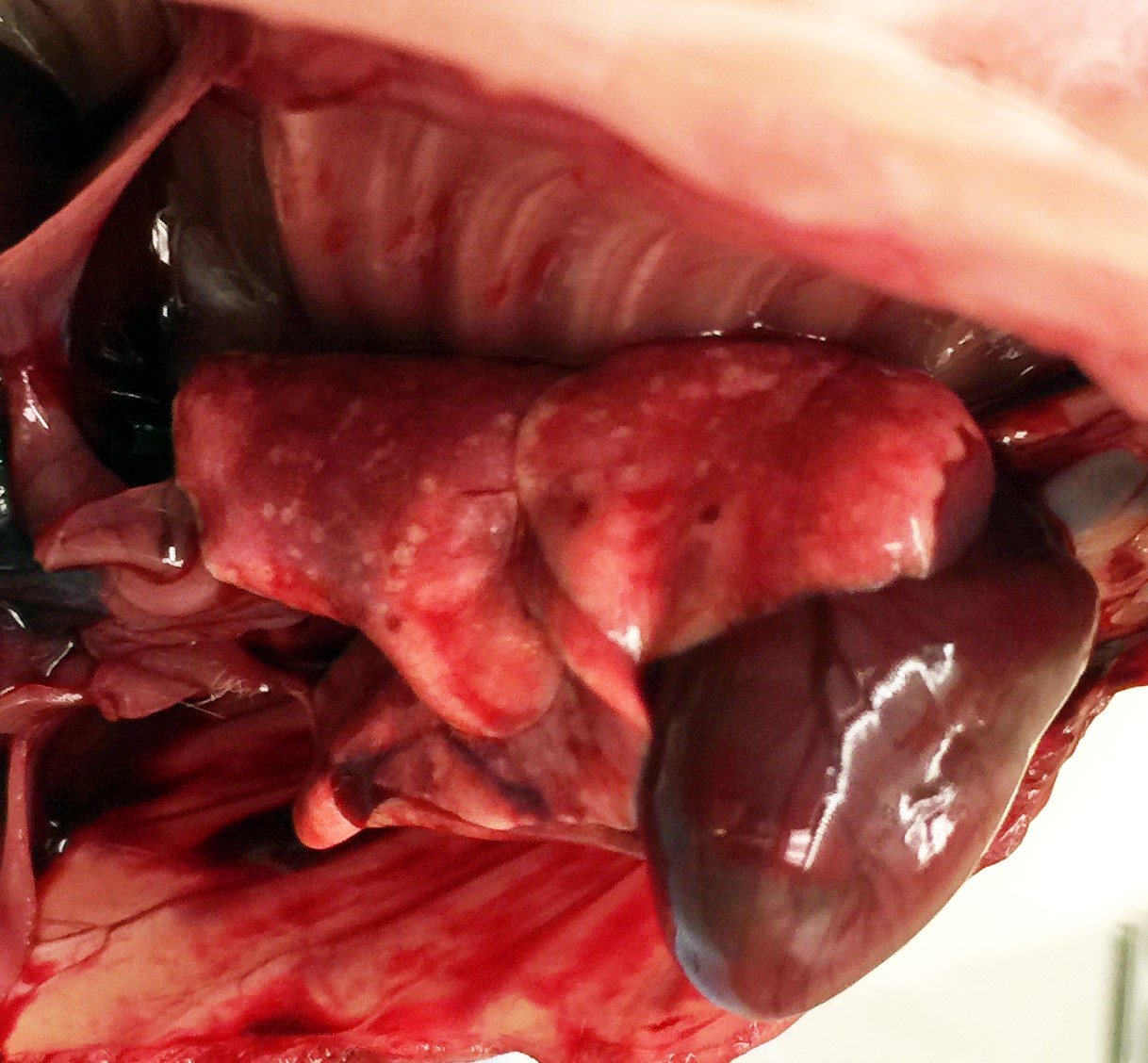
Characteristic gross findings expected for
the X-SCID strain (1) were present in all rats examined and consisted of severe
thymic hypoplasia, unidentifiable lymph nodes, and hypoplastic spleens. All
adult rats had mild crusting of the rostral nasal turbinates and multifocal,
1-2 millimeter diameter, white-tan foci on the pleural surface and fewer within
the parenchyma on cut section. Few similarly-sized red foci were also present
on the pleural surface and throughout the parenchyma.
Histopathologic Description:
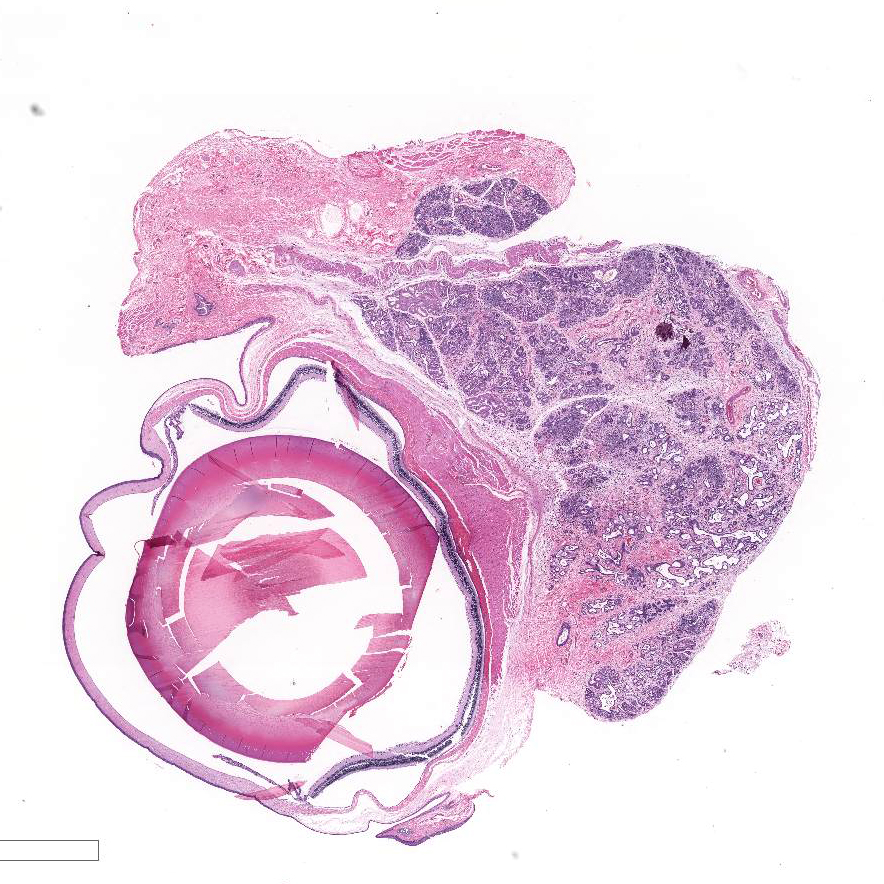
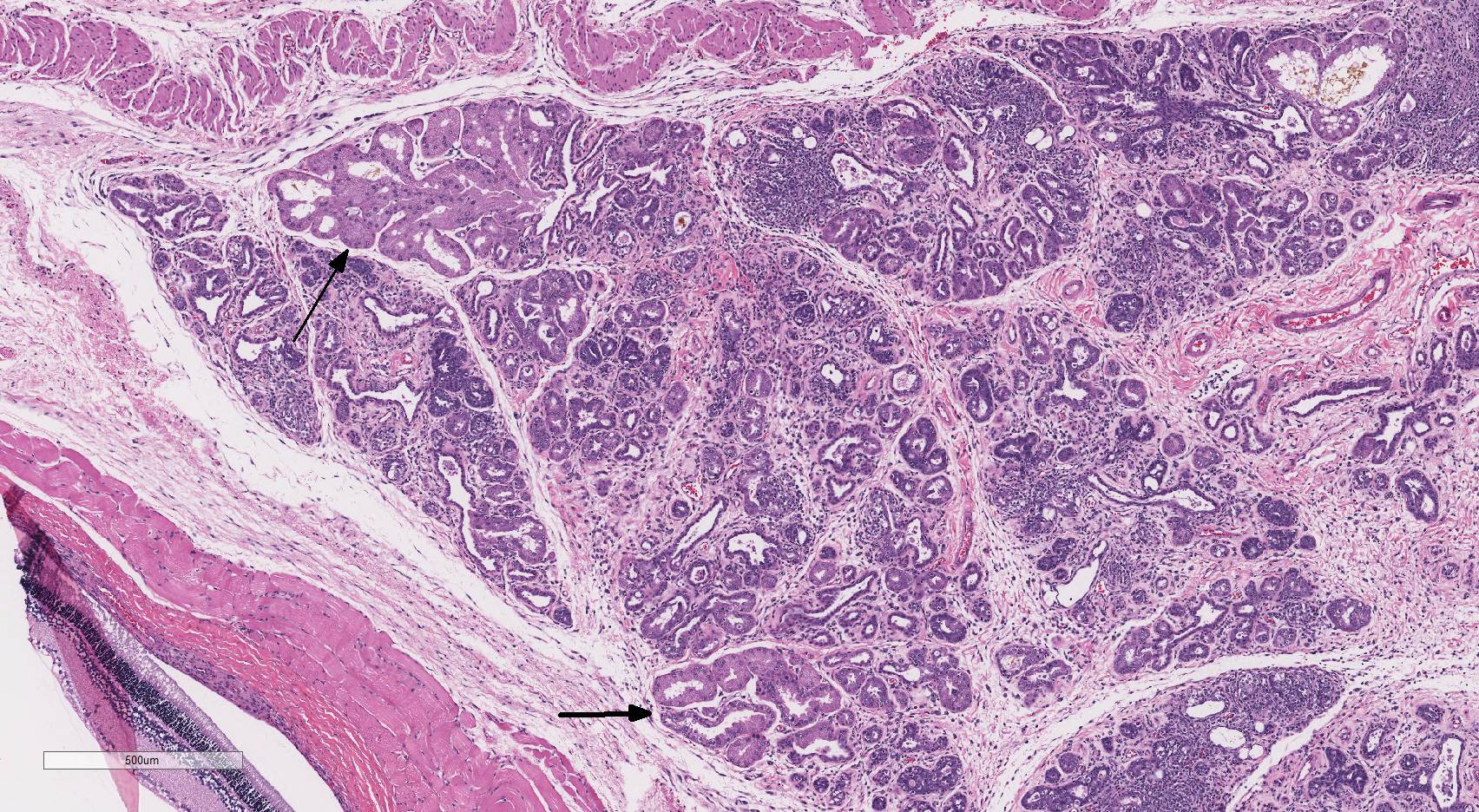
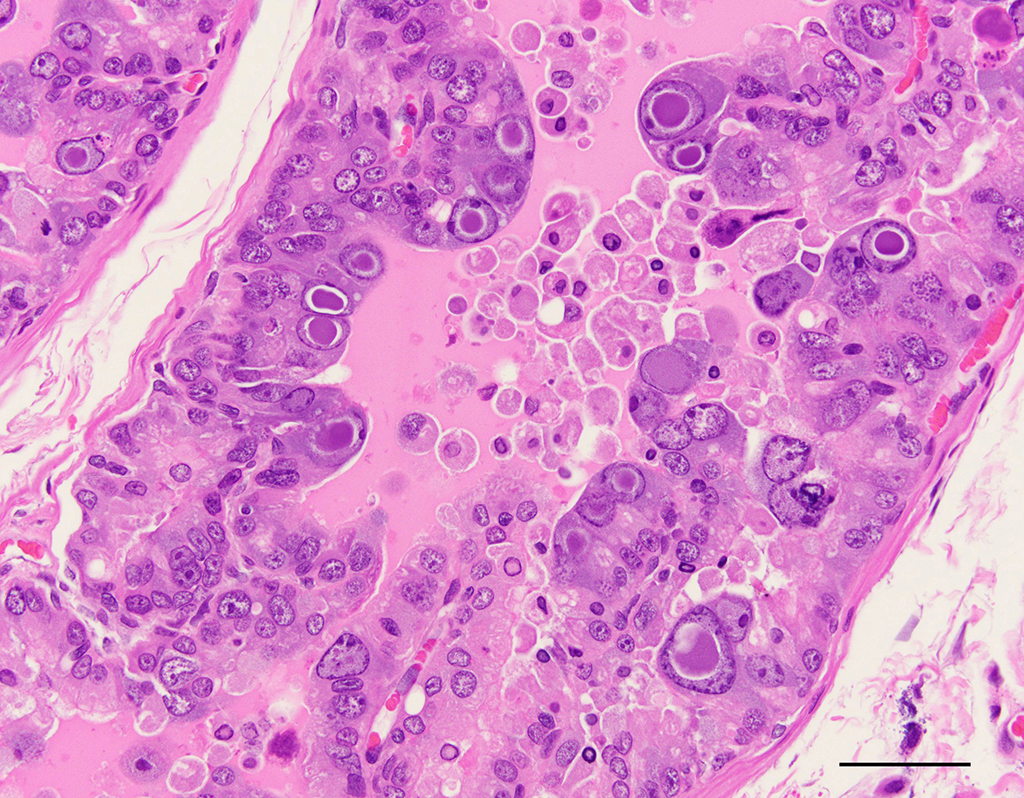
Harderian
gland: There is severe atrophy and loss of the glandular acini with
replacement by fibrous connective tissue. The epithelium of remaining glands is
often necrotic or attenuated and infiltrated by mixed inflammatory cells
composed of lympho-cytes, plasma cells, macrophages, and fewer neutrophils and
mast cells. These inflammatory cells are present surrounding glands, ducts, and
extend into fibrous connective tissue. There are increased numbers of intralobular
and interlobular ducts which are dilated and lined by hyperplastic or
attenuated epithelium and multifocally contain sloughed cells and cellular
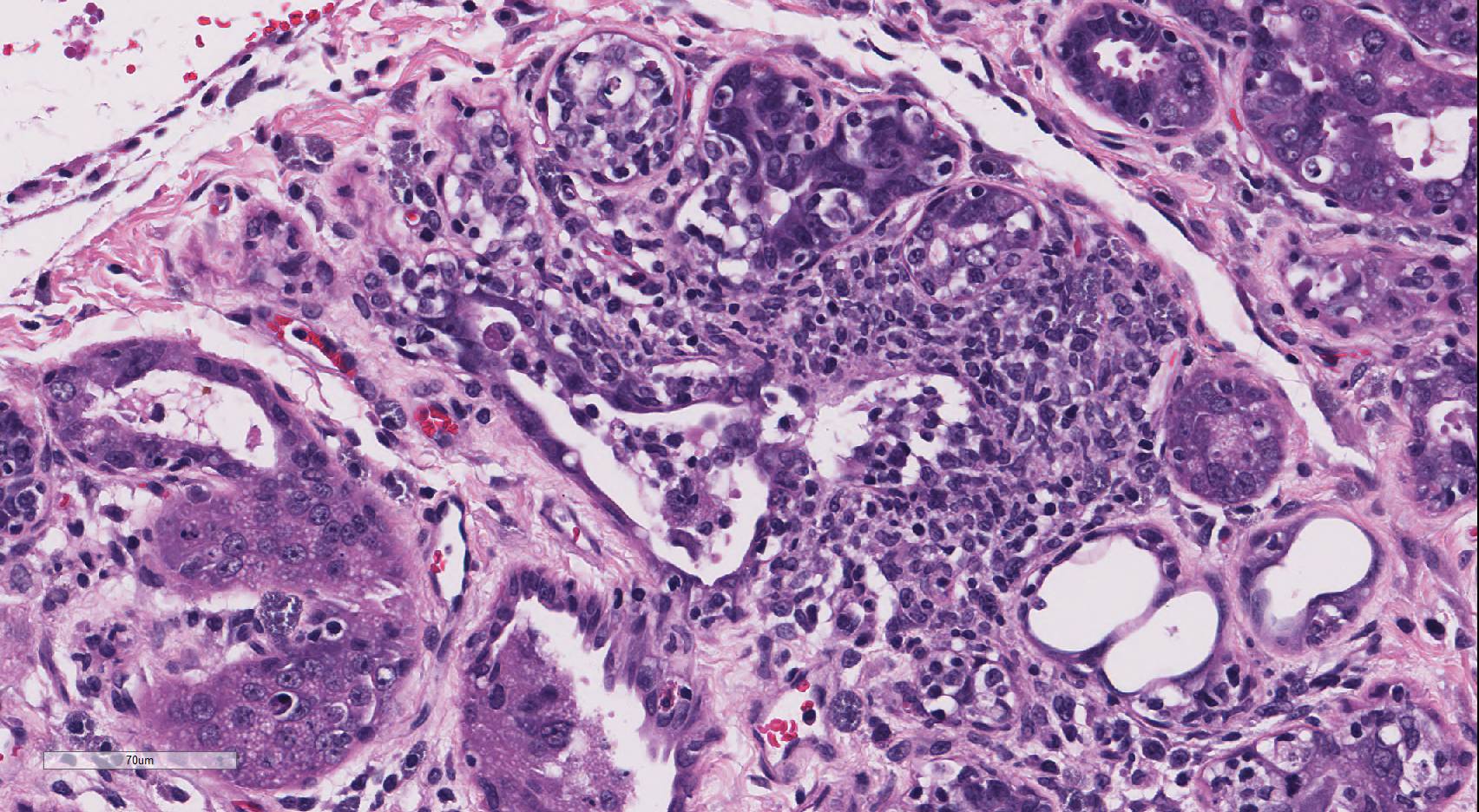
debris. Occasionally, ductal and acinar epithelial cells contain eosinophilic
to amphophilic intranuclear inclusion that are up to 35µm in diameter and are
sometimes surrounded by a clear halo.
Morphologic Diagnosis:
Harderian gland: Acinar atrophy,
diffuse, severe, with fibrosis, lymphoplasmacytic inflammation, ductal
hyperplasia, and epithelial intranuclear inclusion bodies.
Lab Results:
Rats within this colony tested positive for
Pneumocystis
carinii via serology and PCR of nasal swabs.
After
histopathology was performed, additional PCR tests were submitted for rat
cytomegalovirus and mouse adenoviruses 1 & 2. An additional PCR for
polyomavirus was performed with primers designed using a target a region of the
VP1 gene that is partially conserved among polyomaviruses, including those
found in mice and hamsters; all follow-up PCR tests were negative.
Condition:
Acinar atrophy/Rat polyomavirus
Contributor Comment:
Additional histopathologic lesions, including
epithelial necrosis, hyperplasia, dysplasia, and intranuclear inclusion bodies,
were present in the nasal cavity, lung, salivary gland (parotid and
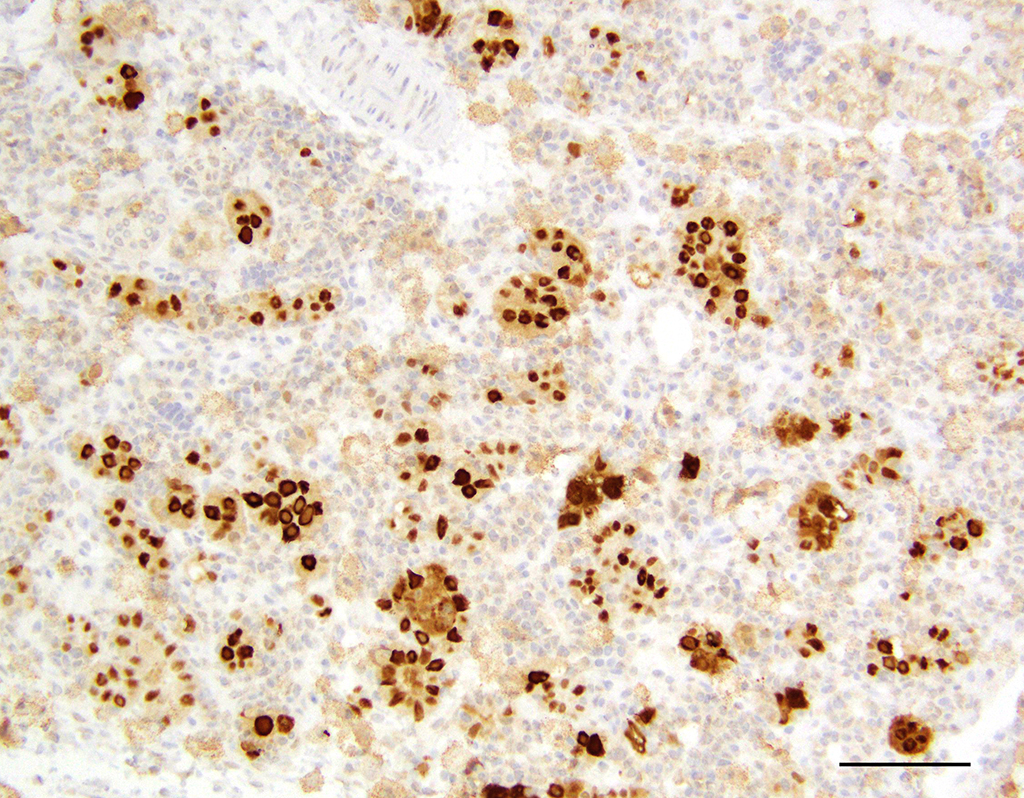
submandibular), prostate gland, and uterus. Immunohistochemistry
of the organs listed above showed strong staining with the pan-polyomavirus
marker, PPIT.
6 The virus was subsequently isolated from the salivary
and Harderian glands of rats within this colony and sequenced. This is a novel
polyomavirus phylogenetically distinct from the rat polyomavirus isolated and
sequenced from feral Norway rats in 2015.
2
Polyomaviruses
(PyVs) are a family of DNA tumor viruses that are known to infect a variety of
mammals, birds, and fish.
1 Most mammalian polyomaviruses cause
subclinical infections with life-long persistence in their natural immune
competent hosts. However, when the host immunity is compromised, the virus can
reactivate and cause disease.
5 Until this discovery, five distinct
PyVs have been identified in rodent hosts: murine PyV, mouse pneumotropic
virus, hamster PyV, Mastomys PyV, and Rat PyV, whose full genomes are available
in the GenBank database.
2
The first
polyomavirus in rats was described as a wasting disease in athymic nude rats by
Ward, et al, in 1984.
7 Inclusion bodies were described in the
salivary glands, Harderian glands, lungs, and nasal glands, similar to those
present in this colony.
7 Also, similar to the previously described
report is the fact that X-SCID rats are severely immune suppressed.
3
This strain has severely hypo-plastic lymphoid organs and markedly decreased T
cells, B cells, and NK cells, making them an excellent model for
xenotransplantation studies.
3 This severe immune suppression makes
them particularly susceptible to viral infections like PyV. It is not yet
clear where these rats were infected with the virus or whether immune competent
rats can be infected, show clinical symptoms, or have histologic lesions when infected with this novel Rat PyV. A
full description of pathology findings and genomic sequencing information for
this novel virus is pending publication (Rigatti and Toptan, et al).
JPC Diagnosis:
Harderian gland: Dacryoadenitis,
necrotizing and histiocytic, chronic, diffuse, moderate to severe, with edema
and occasional epithelial intranuclear inclusion bodies, X-SCID rat,
Rattus
norvegicus.
Conference Comment:
The contributor provides an outstanding description and synopsis of the lesions
of a novel polyomavirus (PyV) infection in an X-SCID rat. Particularly
striking are the characteristic large, prominent epithelial intranuclear viral
inclusions that marginate the chromatin and often enlarge the nucleus; there is
variation among the slides in the number of intranuclear inclusions present. As mentioned by the
contributor, the majority of mammalian PyVs cause subclinical infections with
life-long persistence in immune competent natural hosts, much like
herpesviruses.
4 However, when the host immunity is compromised, such
as in this particular strain of rat, the virus can cause disease.
Polyomaviruses are of particular research interest, and murine PyVs are
used as models of persistent virus infection in human disease.
1,2,3,6
The most well-known human PyVs, BK virus and JC virus, are associated with
severe disease in immunosuppressed human patients; and Merkel cell PyV is associated with Merkel cell carcinoma, a rare and
highly aggressive neoplasm of neuroendocrine cells of the skin.
2 The
virus has long been established as potentially carcinogenic, causing many
different types of tumors in experimental systems, hence the name
poly(many)-oma(tumor)-virus.
Conference participants noted that this case nicely demonstrates cytomegaly, karyomegaly, and glassy intranuclear
inclusions characteristic of PyV infection in the Harderian gland. Many
participants noted that rat cytomegalovirus infection can cause similar
inclusions in the Harderian gland of rats, with large eosinophilic owl-eye
inclusions that marginate the chromatin.
4 However, PyV inclusions
in tissue have a homogenous basophilic or amphophilic appearance, which is
distinct from cytomegalovirus and adenoviral inclusions.
4
Participants also discussed that sialodacryoadenitis virus, a highly contagious
betacoronavirus, which can cause similar lesions in the Harderian gland of
rats; however, that virus does not result in the formation of intranuclear
inclusions.
4
Attendees discussed
some other significant PyVs of veterinary importance, including simian virus 40
(SV40) which caused progressive multifocal leukoencephalopathy in
immunosuppressed rhesus macaques; the
Mesocricetus auratus PyV1 which
induces trichoepithelioma and lymphoma in hamsters; the K virus and murine
pneumotropic virus in mice;
Procyon lotor PyV1 which causes high-grade
neuroglial olfactory tumors in raccoons; Aves PyV1 that results in budgerigar
fledgling disease in psittacine birds; and goose hemorrhagic PyV1, the cause of
hemorrhagic nephritis and enteritis in anseriform birds.
1-7
The conference moderator cautioned participants
that, while inclusions present in the intra-orbital Harderian gland are due to
viral infection, pseudoinclusions and syncytial cells in the exorbital lacrimal
gland are part of its normal anatomy and should not be confused for viral
cytopathic effect. In addition, participants noted numerous mast cells within
the interlobular connective tissue, which is also a normal finding in rats. The
moderator further observed that within the adjacent eye the retinal epithelium
lacks pigment, indicating that this rat is an albino. As a result of the lack
of pigmentation, albino rats are much more susceptible to retinal degeneration
and cataract formation induced by ultraviolet light as compared to normally
pigmented animals. Degenerative changes may also occur in the Harderian glands
of rats exposed to high-intensity lights.
4
References:
1. Buck CB, Van
Doorslaer K, Peretti A, Geoghegan EM, Tisza MJ, An P, Katz JP, Pipas JM,
McBride AA, Camus AC, McDermott AJ, Dill JA, Delwart E, Ng TF, Farkas K, Austin
C, Kraberger S, Davison W, Pastrana DV, Varsani A. The ancient evolutionary
history of polyomaviruses.
PLos pathogens. 2016; 19:12(4):e1005574.
2. Ehlers B,
Richter D, Matuschka FR, Ulrichd RG. Genome sequences of a rat polyomavirus
related to murine polyomavirus,
rattus norvegicus polyomavirus 1.
Genome
Announc. 2015; 3(5):e00997-15.
3. Mashimo T,
Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of
knockout rats with x-linked severe combined immunodeficiency (X-SCID) using
zinc-finger nucleases.
PLoS one. 2010; 5(1):8870.
4. Percy DH,
Barthold SW. Rabbit. In:
Pathology of Laboratory Rodents and Rabbits,
4th ed., Ames, IA: Blackwell Publishing; 2016:122,161.
5. Stevens H,
Bertelsen MF, Sijmons S, Van Ranst M, MaesP. Characterization of a Novel
Polyomavirus Isolated from a Fibroma on the Trunk of an African Elephant (
Loxodonta
africana).
PLoS one. 2013; 8(10):1-9.
6. Toptan T, Yousem
SA, Ho J, Matsushima Y, Stabile LP, Fernández-Figueras MT, Bhargava R, Ryo A,
Moore PS, Chang Y. Survey for human polyomaviruses in cancer.
JCI Insight.
2016; 1(2):85562.
7. Ward JM, Lock A,
Collins Jr MJ, Gonda MA, Reynolds CW. Papovaviral sialoadenitis in athymic nude
rats.
Lab Animals. 1984; 18:84-89.