Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 14
15 January 2020
Dr.
Neel Aziz
Supervising Veterinary Pathologist
Smithsonian Conservation Biology Institute
National Zoological Park
Washington DC, 20008
CASE IV: 016-045846 26 (JPC 4101490).
Signalment: A 10 year-old, female intact, Red Kangaroo (Macropus rufus)
History: A ten year old, intact, female Red Kangaroo (Macropus rufus) was submitted for necropsy from a local zoo. The animal had presented acute dyspnea two days prior to death, and radiography was performed revealing extensive mixed pulmonary pattern. A large mass in the right inguinal area was noted, and the mammary gland was slightly enlarged. The animal was unresponsive to supportive care, including fluid therapy, antibiotics and analgesics, among others. Three years before, the animal had an arthrodesis and stem cell therapy in the right hock due to severe traumatic injury. Also, a wallaby peer from the same enclosure had died from gastrointestinal histoplasmosis recently.
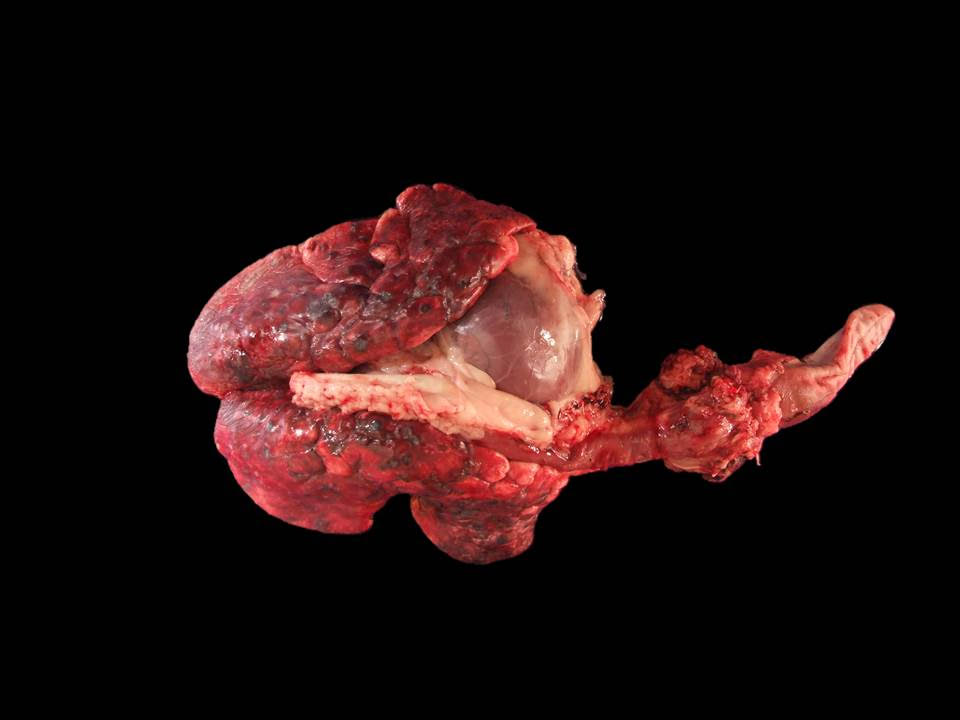
Gross Pathology: The right axillary lymph node was moderately enlarged with a large amount of surrounding fat. On cut section, it was cavitated and contained serosanguinous fluid. The subcutis in the left inguinal area had a focally extensive area of hemorrhage, measuring 2 x 4 x 5 cm (biopsy-induced, as per the history in the submission form) with an underlying, thickly encapsulated, 2 cm in diameter, round mass with a solid, dark brown, necrotic center. The right inguinal lymph node was markedly enlarged, with a cavitated, straw-colored fluid-filled center. All other lymph nodes examined were dark-red, cavitated and fluid-filled. Bilaterally, the caudal nipples were diffusely enlarged and blue (cyanotic). The left caudal mammary gland was diffusely and markedly enlarged and firm, measuring 9 x 5 x 3 cm 2 x 0.7 x 3 cm, with two additional lobules measuring 4.5 x 3.5 x 2 cm and 2 x 1.5 x 1 cm. On cut section, they were cavitated, filled with serosanguinous fluid and a solid, tan area that contained a focal, necrotic center.
The thoracic cavity contained 130 mL of straw-colored, cloudy fluid. Affecting about 75 % of the pulmonary parenchyma, there were multifocal to coalescing, variably sized, dark red to tan, firm nodules, some of them with an umbilicated center. The pleura was diffusely thickened and cloudy. Adjacent to the right ovary and corresponding with the right lumbar lymph node, there was an approximately 6.5 x 3.5 x 3 cm, multilobulated, firm, tan mass firmly adhered to the caudal vena cava at the iliac bifurcation. The endothelium of the caudal vena cava at this site had multifocal 1 mm in diameter, firm nodules. On cut section, the mass was solid tan, with a brown, necrotic center. The pelvis of the right kidney was markedly distended with moderate atrophy of the renal medulla. Along the urinary bladder, following the ureteral insertion, there were multifocal, 4 mm in diameter, firm, tan nodules situated in a linear pattern.
Laboratory results: N/A.
Microscopic Description:
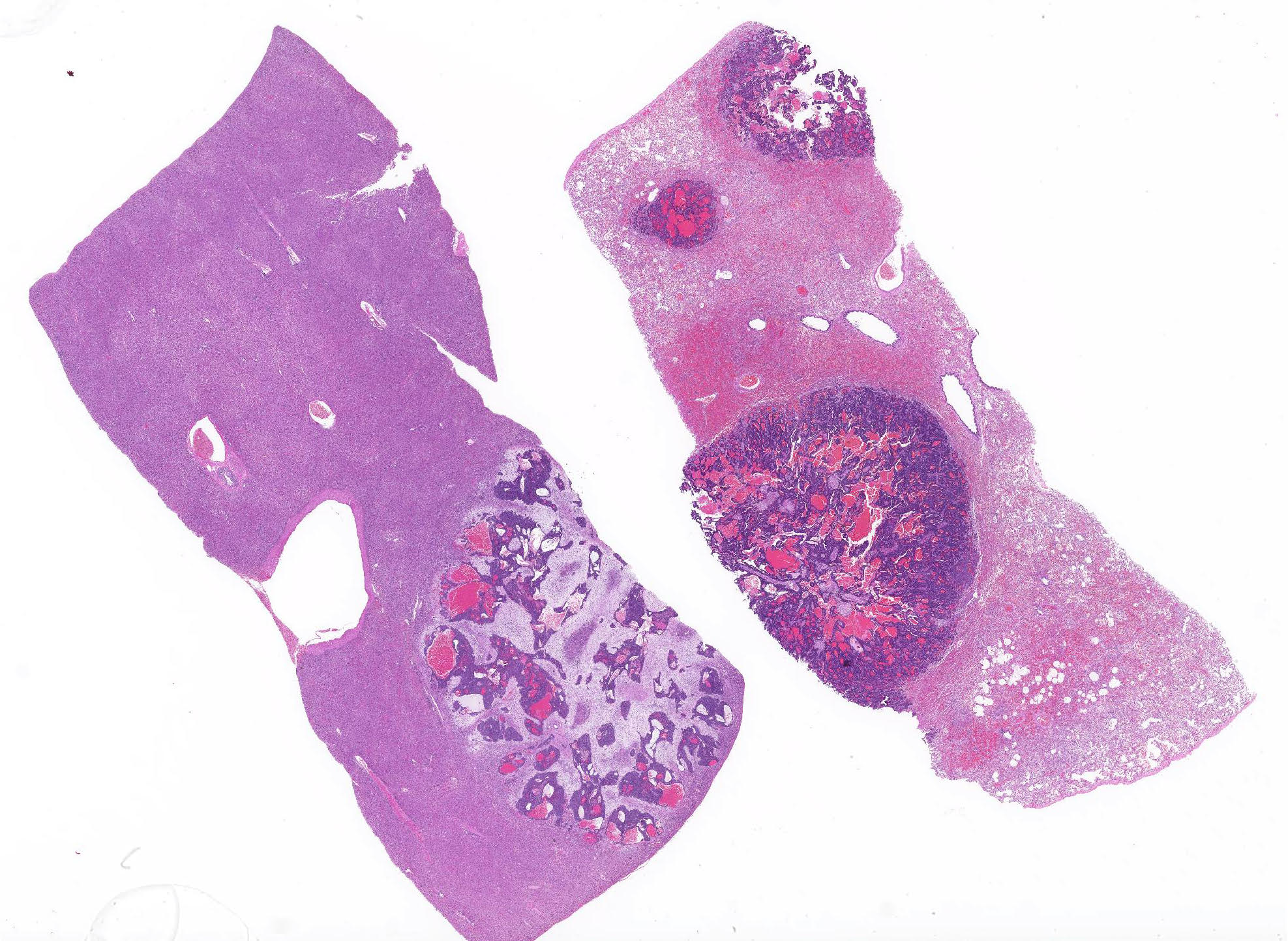
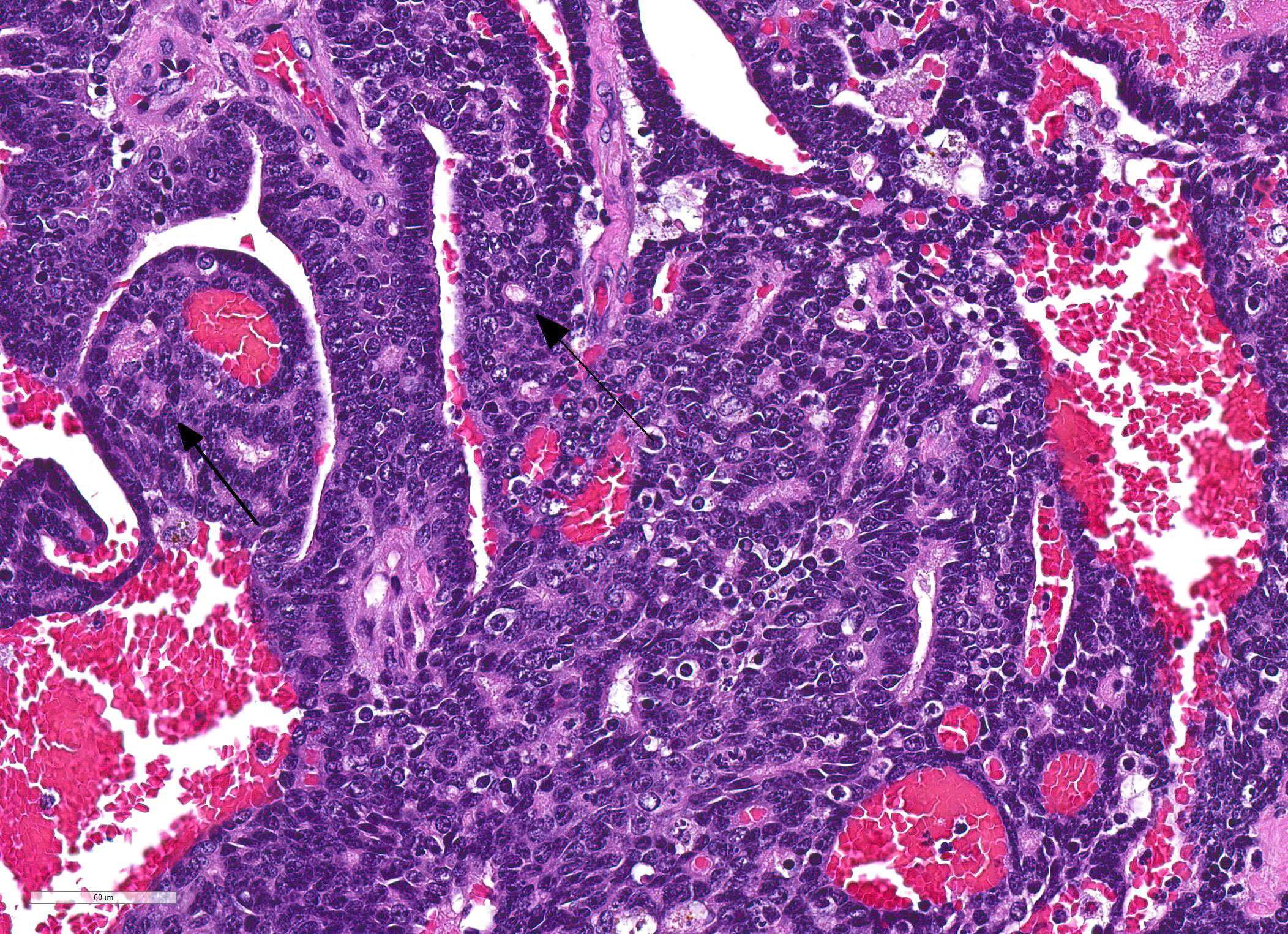
Lungs: Affecting approximately 70% of the sections examined, the parenchyma is effaced, replaced and compressed by a well-demarcated, unencapsulated, moderately cellular neoplasm arranged in acini, nests and tubules supported by a thick fibrovascular stroma, and intermixed with variably sized areas of hemorrhage and congestion. The neoplastic cells are polygonal, with variably distinct cell borders, a scant, finely vacuolated, eosinophilic cytoplasm, and a single, round to oval, vesicular nuclei with up to two distinct basophilic nucleoli. There are marked features of anisocytosis and anisokaryosis with occasional binucleated cells, and there are seven mitotic figures per ten 600X fields examined. The mitotic figures are occasionally multipolar and bizarre. Frequently, the ductules formed by the neoplastic cells are filled with red blood cells, and foamy macrophages that often contain intracytoplasmic, brown, granular pigment (hemosiderin). The remaining alveolar spaces are either markedly compressed, congested or filled with an extracellular, eosinophilic fibrillar material (fibrin), hemorrhage and increased numbers of alveolar macrophages.
Liver: Affecting around 30% of the sections examined, the hepatic parenchyma is effaced, replaced and compressed by the same neoplastic population as described for the lungs. Occasionally in the portal triads, there are neoplastic cell emboli within the lumen of the vessels and there is a mild to moderate, biliary duct hyperplasia. The remaining hepatocytes, especially those in proximity of the neoplasm, contain a golden brown, granular pigment (suspected bilirubin) and several intracytoplasmic, clear vacuoles that occasionally push the nuclei eccentrically (vacuolar degeneration). The sinusoidal spaces are diffusely and mildly congested.
Contributor Morphologic Diagnosis:
Lungs and Liver: Mammary carcinoma, metastatic.
Contributor Comment: Other tissues affected that were histologically evaluated include right and left mammary glands, inguinal and lumbar lymph nodes, liver, lung and adrenals. Many sections had neoplastic cells as neoplastic emboli or closely associated with the vasculature. The lumbar lymph node was markedly enlarged, compressing the adjacent ureter causing unilateral hydroureter and hydronephrosis.
Immunohistochemistry for vimentin, cytokeratin (AE1/AE3) and chromogranin, as well as Grimelius staining for argyrophilic granules were performed to rule-out a neoplasm of non-mammary origin, such as neuroendocrine cells as suggested by occasional ?pseudorosette-like? structures within the neoplasm. The neoplastic cells were strongly positive for cytokeratin, and the surrounding scirrhous reaction was vimentin positive. All other immuno- and histochemical-staining performed were negative (no internal control nor validation for a red kangaroo was performed for chromogranin immunohistochemical staining).
The mammary glands are classified as mammalian-specific modified subcutaneous apocrine sweat glands of ectodermal and mesodermal origin with a function of new-born nourishment and passive immunity. 2 Neoplasms are infrequently reported in Macropodidae, with little literature available regarding mammary adenocarcinomas in Red Kangaroos (Macropus rufus) which according to few studies, is the most common neoplasm along with oral squamous cell carcinomas in this species. 5 However, mammary neoplasms are exceedingly common in dogs, cats and humans. 2 In dogs, which has the highest incidence among domestic species, most mammary tumors are clinically benign regardless of their phenotype. 1, 4 Mammary carcinomas are very heterogeneous and complicated, creating increasingly intricate classification schemes in order to characterize them. Nevertheless, simple, solid and complex carcinomas are the most prevalent type reported in the dog, with variable survival times but low metastasis. 4 It must be pointed that cytology and metastasis potential, most commonly via lymphatics, does not correlate well.4 Dogs have a higher incidence of mammary neoplasm development in the caudal glands, while cats do not have a predilection site within the mammary chain. 4
Generally, six main features are characterized as prognostically important in mammary tumors, which include metastasis to draining lymph node, intravascular tumor emboli, peripheral invasion, unique histological phenotype, histological grade (dysplasia and mitotic rate), and tumor size. In general, mammary carcinomas in cats with a size more than 3 cm in diameter have poorer prognosis, with shorter survival time.1,2,4 In addition, less differentiated carcinomas have a greater metastasis chance and therefore, have shorter survival time. Many risk factors are associated in the development of mammary neoplasms in dogs and cats, such as age, reproductive status, exogenous hormone exposure, breed susceptibility, diet, and obesity.
Grading schemes for carcinomas, using an adaptation of the Elson and Ellis/Nottingham method for human breast carcinomas are available, although there are few cases with good follow-up. This scheme is based on the degree of tubular formation, nuclear pleomorphism, and mitotic index. 1,2
According to the limited literature available, mammary tumors are one of the most common neoplasms in Red Kangaroos (Macropus rufus) and exceedingly common in dogs, cats and humans, with a heterogeneous, and complicated classification and characterization, but frequently malignant in the feline and benign in the canine population.
Contributing Institution:
Kansas State University-Veterinary Diagnostic Laboratory and the Department of Diagnostic Medicine/Pathobiology, Manhattan, KS 66506. http://www.ksvdl.org/
JPC Diagnosis: Liver, lung: Carcinoma, metastatic.
JPC Comment: The attendees agreed that without the history or a mammary mass, it would be difficult to identify the multiple metastatic foci as of mammary origin. We agree with the contributor?s differential diagnosis of neuroendocrine carcinoma based on the morphology of the neoplastic cells as well as the tendency to form rosettes or rosette like structures within the submitted sections. In concurrence with the contributor?s findings, a JPC-run chromagranin and synaptophysin were both negative in this case.
As mentioned by the contributor, neoplasia in kangaroos is likely underreported, but also appears to be relatively uncommon based on available literature. There are no previous submssions of neoplasia in kargaroos in the WSC, and no mentions of macropod neoplasia in the recently published Pathology of Wildlife and Zoo Animals, edited by Terio et al. In a 2007 report on 28 kangaroos autopsied in a major metropolitan zoo in the 1990?s, six developed neoplastic disease, two of which were mammary neoplasia (additionally, two developed oral squamous cell carcinoma, with one lymphoma and one gastric lipoma). A review of the available literature at the time, revealed a similar incidence of reports of mammary gland adenocarcinoma (3/17) and oral cavity (3/17).
Using the tammar wallaby as a model, the mammary gland of the marsupial has recently been investigated by Khalil et al. and shows significant difference from other mammals, likely because of the type of young (extremely immature and almost fetal in nature) that it bears following an extremely short gestation (26.5) day gestation.. Pouch young remain attached to the teat for 100 days, where upon they detach and suckle periodically for up to an additional 110 days in the pouch. Marsupials have developed a lactation strategy consistent with these distinct periods of nursing. In the phase of lactation during permanent attachment of the altricial yound, the milk is high in complex carbohydrates and low in fat and protein. Following detachment from the nipple during a period of rapid growth, eventual exit of the joey from the pouch, and a gradual involution of the mammary gland, the composition of the milk changes to a high protein, low carbohydrate composition, In addition, a number of novel milk proteins inclding SOD3 which protects agains mammary gland toxicity, as well as a number of other proteins which protect the mammary gland from potential infection during a period of milk stasis.
References:
1. Foster RA. Female Reproductive System and Mammae. In:Zachary JF Pathologic basis of Veterinary Disease, 6th ed. 2016:1191-1192
2. Goldschmidt MH, Pe?a L, Zapulli V. Tumors of the Mammary Gland. In; Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. 2016: 723-765
3. Keller, AK , Nevarez JG, Rodriguez D, Gieger T, Gumber S Diagnosis and Treatment of Anaplastic Mammary Carcinoma in a Sugar Glider (Petaurus breviceps) Journal of Exotic Pet Medicine 2014; 23(3): 277-282
4. Schlafer DH, Foster RA. Female Genital System. In:Maxie M. G. Jubb, Kennedy and Palmer?s Pathology of Domestic Animals, 6th ed; 3: 460-463.
5. Suedmeyer W, Johnson G. Survey of neoplasia in red kangaroos (Macropus rufus), 1992-2002, in a zoological collection Journal of Zoo and Wildlife Medicine 2007; 38(2):231-239.