Conference 22, Case 4
Signalment:
2-month-old, female, Toggenburg goat (Capra aegagrus hircus)
History:
Presented with clinical complaints of vomiting and lethargy. Huge amount of fluid in peritoneal cavity (ascites). No abnormalities found on echocardiac examination and blood examination. Laparascopy performed: suspicion of encapsulating peritoneal sclerosis. Died at night.
Gross Pathology:
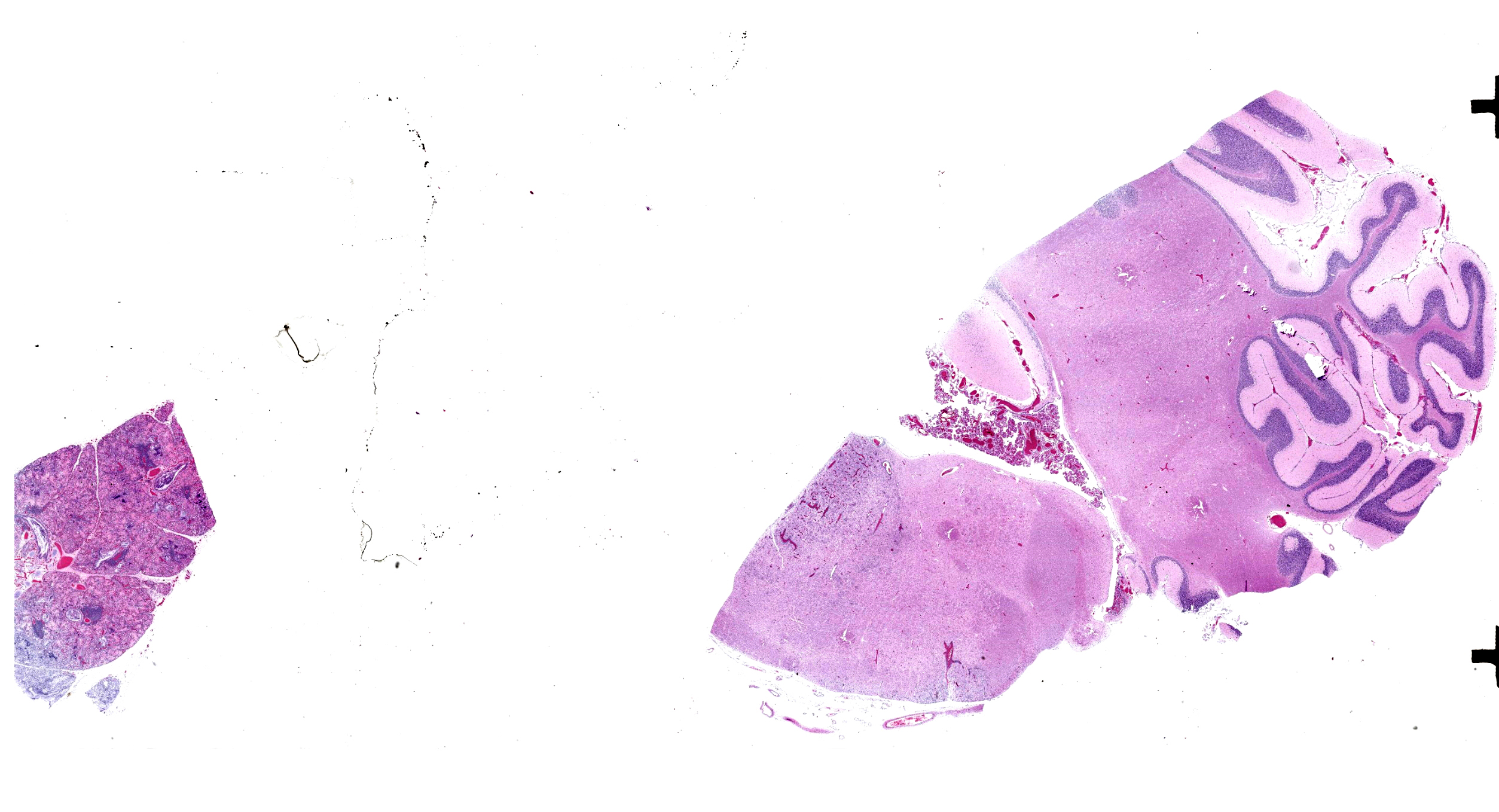
A 2-month-old female Toggenburg goat kid presented for nystagmus, incoordination, and inability to ambulate. Several days prior, this kid’s sibling was euthanized for similar severe neurologic signs.
Laboratory Results:
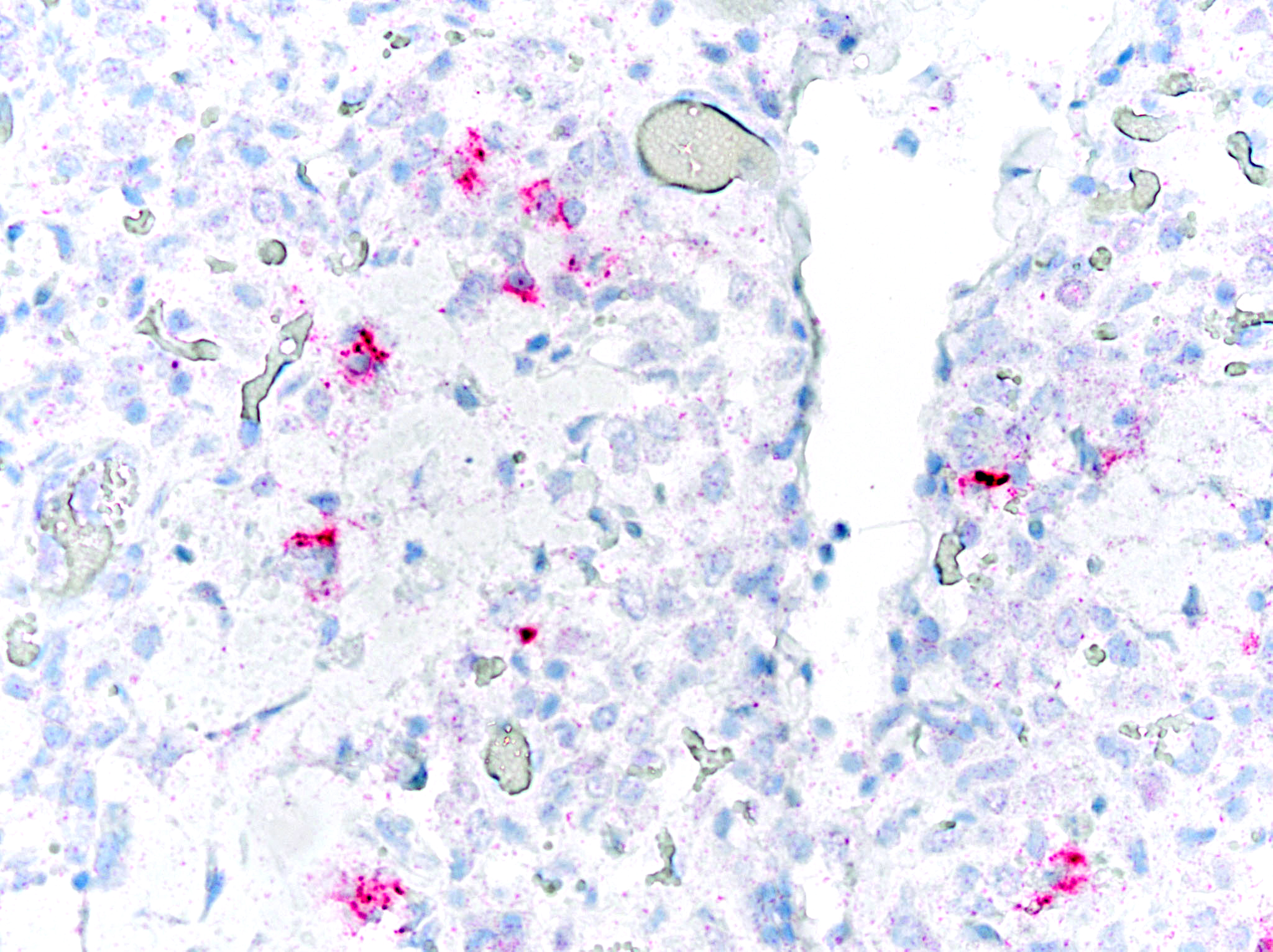
Immunohistochemical staining for Caprine Arthritis and Encephalitis Virus was positive on brainstem and lung tissue (Michigan State University, Veterinary Diagnostic Laboratory).
Aerobic culture grew few colonies Streptococcus infantarius, Streptococcus equinus, and coagulase negative Staphylococcus spp. Mycoplasma spp. PCR was negative. (Ohio Department of Agriculture, Animal Disease Diagnostic Laboratory).
Microscopic Description:
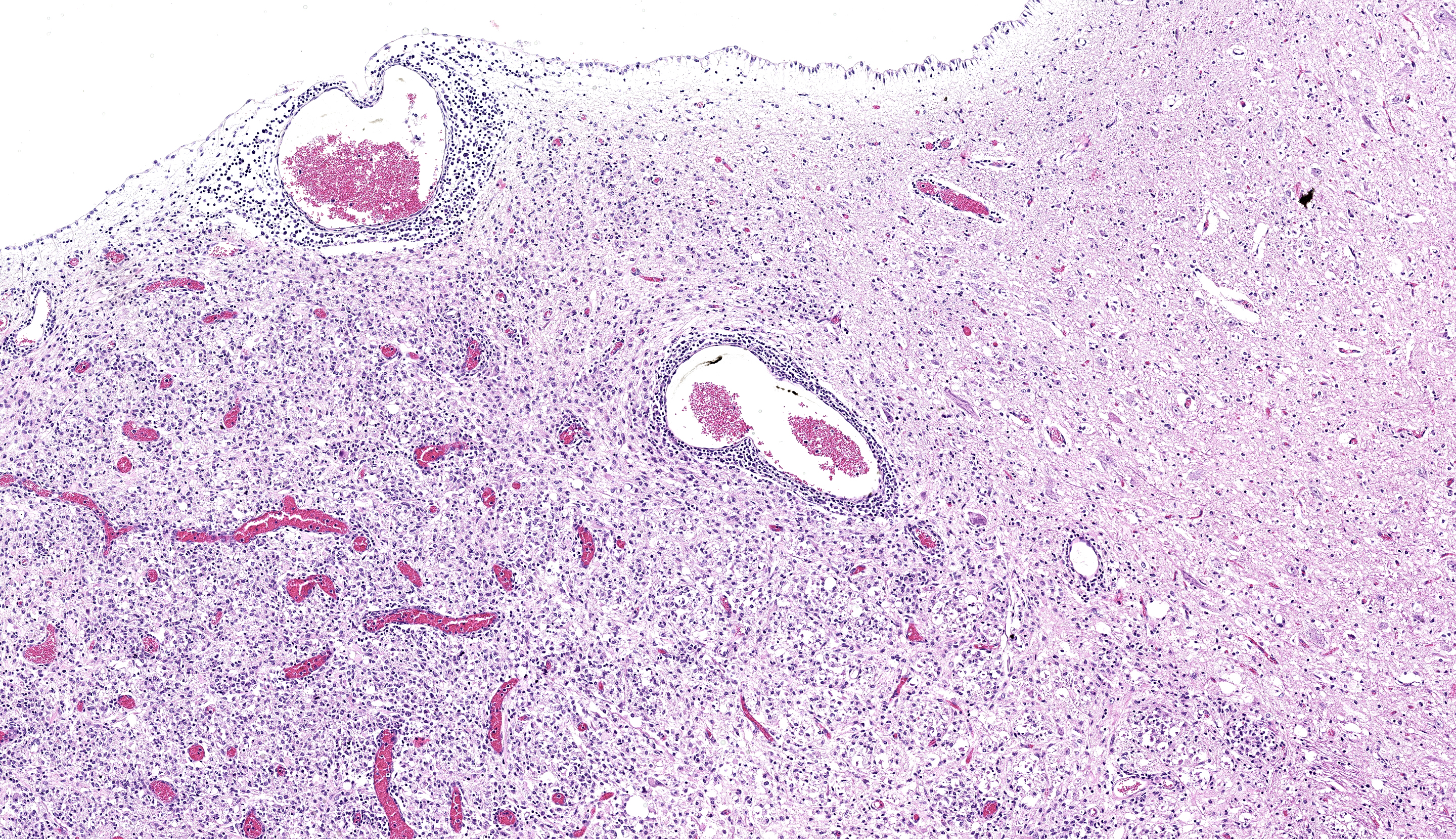
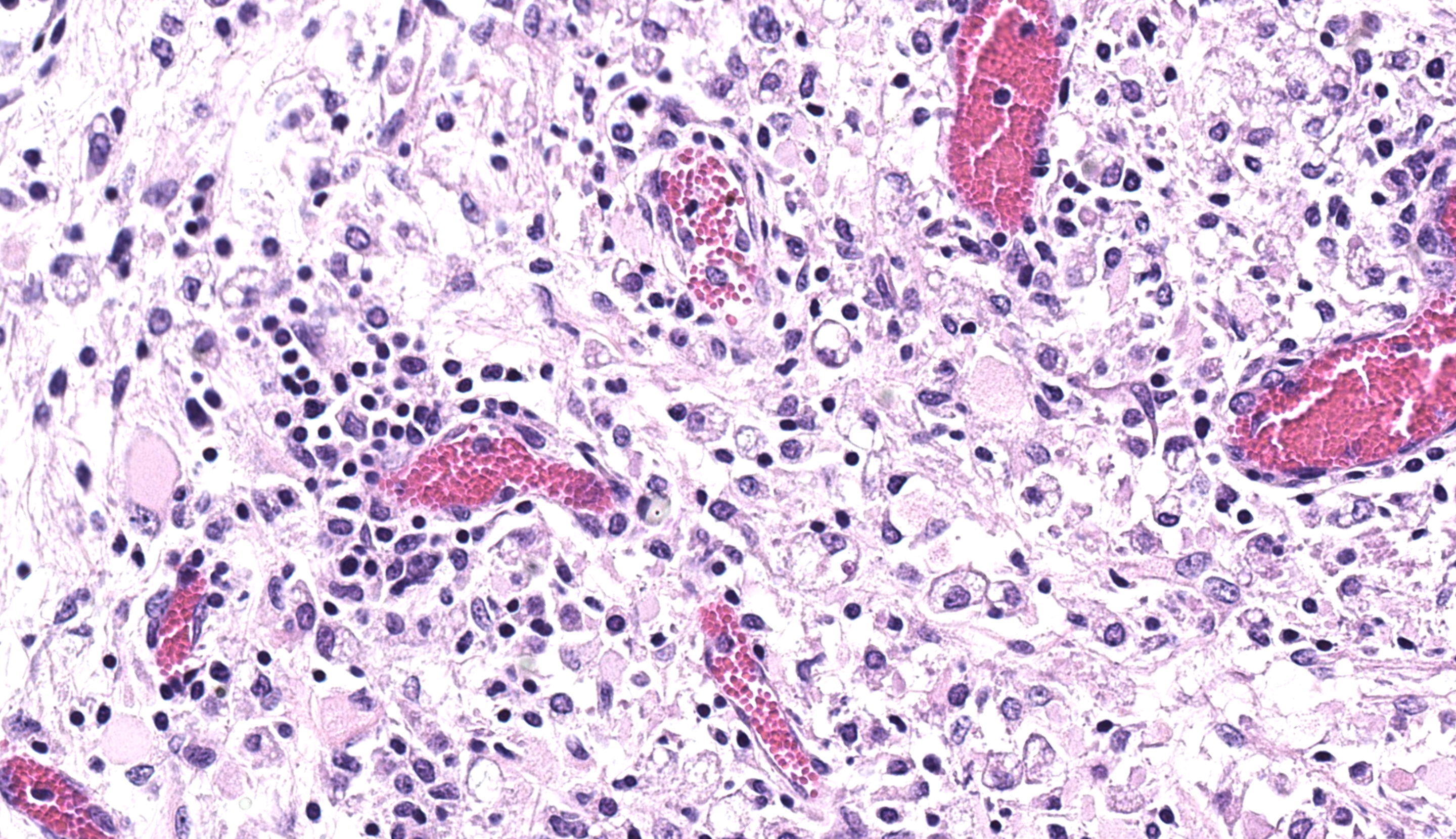
Brainstem and cerebellum: Regionally affecting the brainstem white matter along the midline is an area of neuropil loss and replacement by numerous macrophages with foamy eosinophilic cytoplasm with eccentrically placed nuclei (gitter cells) and mild to moderate numbers of reactive astrocytes, microglia, and admixed lymphocytes and fewer plasma cells. Myelin sheaths are frequently replaced by gitter cells and are rarely dilated with either swollen hypereosinophilic axons (spheroids) or macrophages (digestion chambers). Scattered throughout the affected region is abundant eosinophilic cellular debris. Virchow Robin’s spaces are multifocally expanded by numerous lymphocytes, histiocytes, and fewer plasma cells (perivascular cuffs).
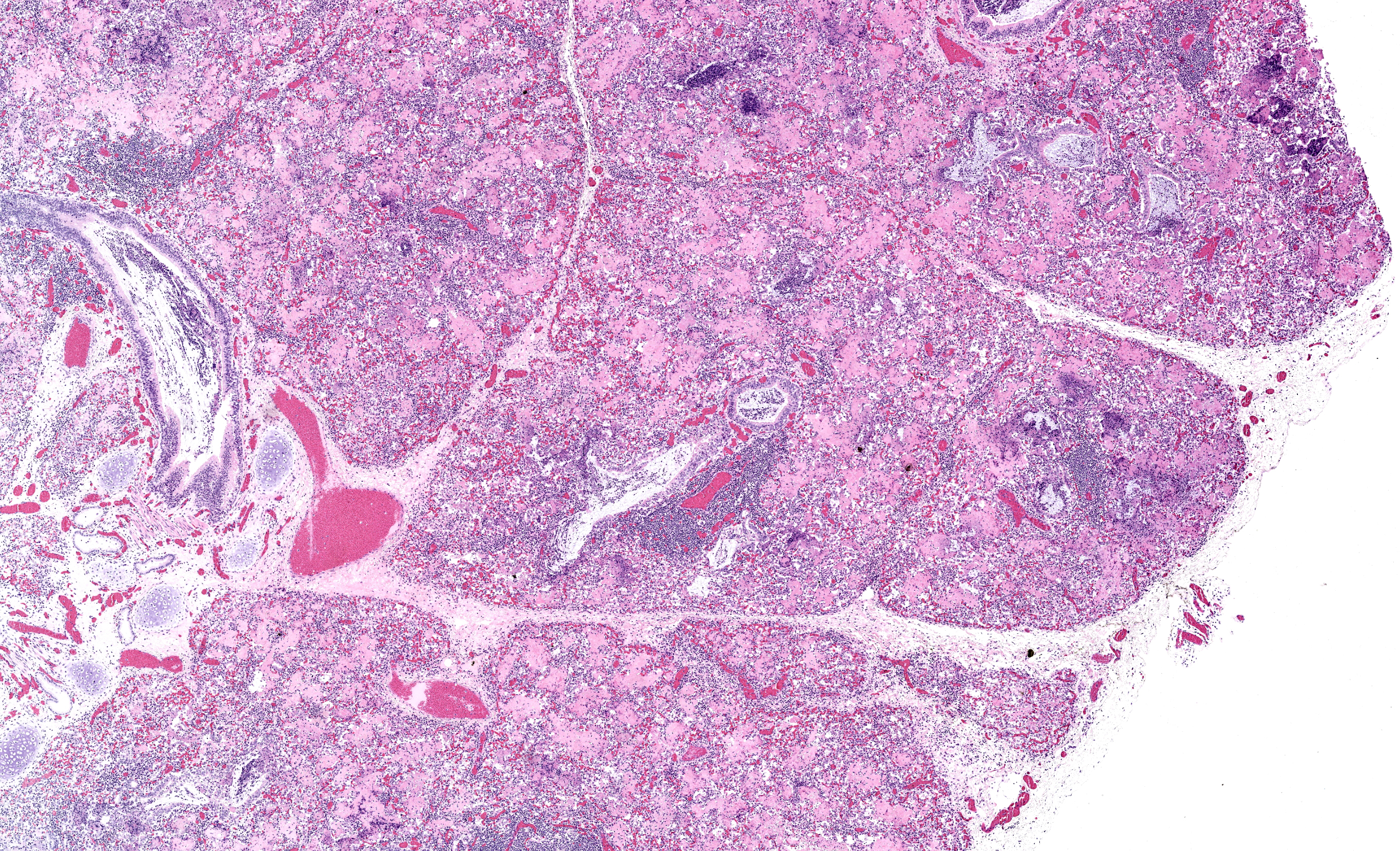
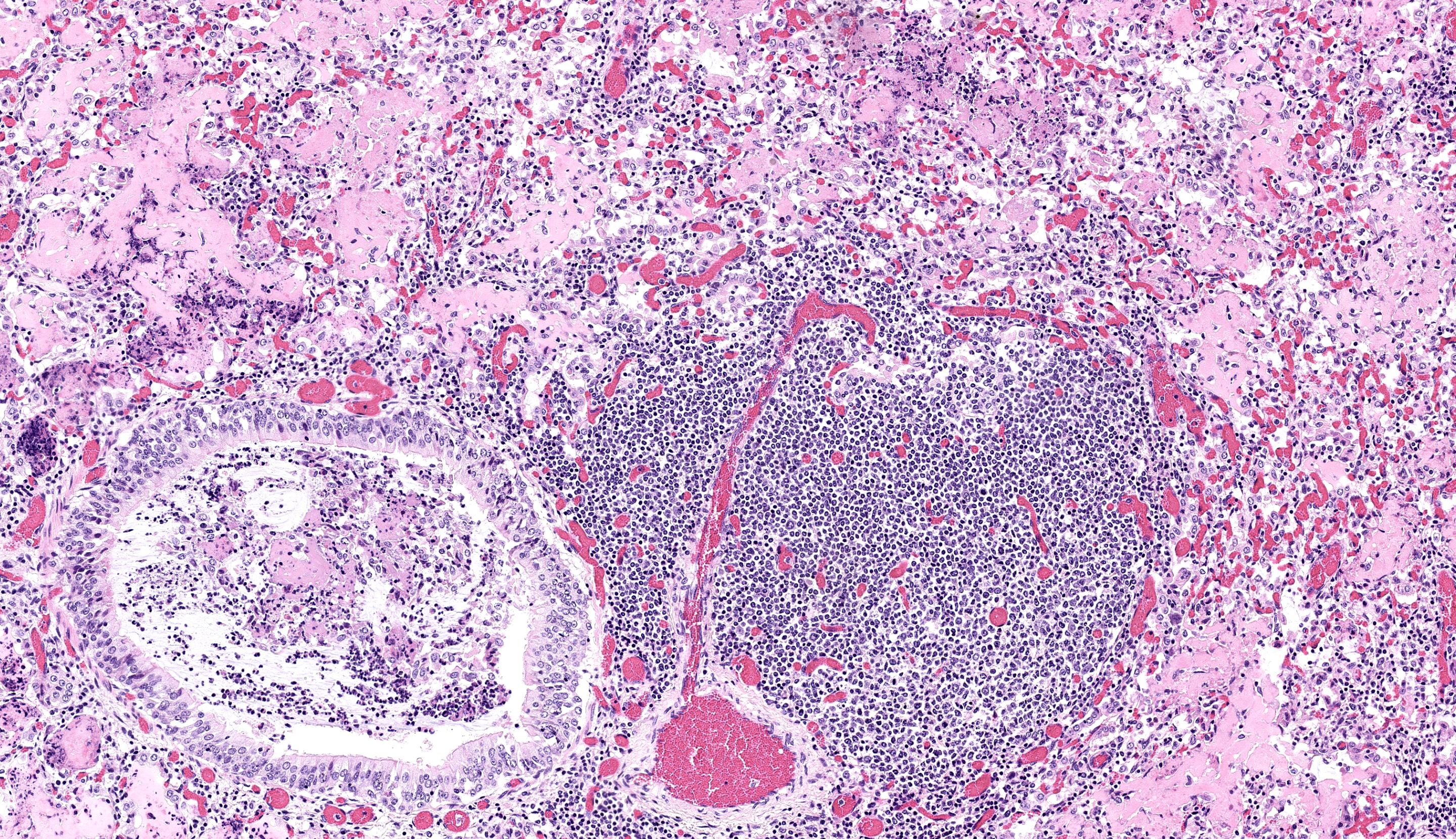
Lungs: Diffusely alveoli are filled with large amounts of eosinophilic amorphous proteinaceous fluid (alveolar proteinosis) and multifocal admixed foci of necrotic cellular and nuclear karhyorrhectic debris. Multifocally, alveoli are lined by plump type II pneumocytes (type II pneumocyte hyperplasia). Vari ably, alveolar septa are expanded by moderate numbers of lymphocytes, histiocytes and fewer plasma cells. Bronchi and large bron chioles contain abundant degenerate neutrophils admixed with eosinophilic cellular and karyorrhectic debris and moderate amounts of basophilic mucin. Lymphoid tissue surrounding bronchi and bronchioles is moderately hyperplastic, with frequent prominent germinal centers.
Contributor’s Morphologic Diagnosis:
Brainstem: Severe, focally extensive, lymphohistiocytic leukoencephalitis with axonal degeneration and neuropil necrosis
Lungs:
- Marked, diffuse, chronic, lymphohistiocytic interstitial pneumonia with type II pneumocyte hyperplasia and alveolar proteinosis
- Moderate, multifocal, acute, suppurative bronchopneumonia
Contributor’s Comment:
Brainstem lesions, interstitial pneumonia, and immunohistochemistry results in this case are consistent with small ruminant lentivirus infection (SRLV), or Caprine Arthritis and Encephalitis Virus (CAEV).
CAEV is a lentivirus within the Retrovirus family. CAEV and Maedi-Visna virus (MV), also called Ovine Progressive Pneumonia, are together referred to as the small ruminant lentiviruses (SRLV).4 Previously CAE and MV were considered species-specific, with CAEV affecting goats and MV affecting sheep; however, several studies have documented cross-species and co-infections demonstrating both species are susceptible to each virus.1, 7
Other prominent lentiviruses include Equine Infectious Anemia Virus, Bovine Immunodeficiency Virus, and Feline Immunodeficiency Virus in domestic species and Human Immunodeficiency Virus in humans. In contrast to these other lentiviruses, SRLVs are unique in that they do not cause immunodeficiency in infected animals.3, 6 The mechanism by which SRLVs are able to evade the host immune system despite an appropriate immune response is not fully understood. One proposed mechanism is that tropism of SRLVs for monocytes, macrophages, and dendritic cells allows the virus to evade the host immune system and disseminate systemically.6 Once in target tissues, the virus can infect other cell types including microglia, endothelial cells, fibroblasts, and epithelial cells, though replication is restricted in these cells.1, 3, 5 These additional tissue targets, particularly mammary epithelium, likely serve as important reservoirs of infection.1, 5
Transmission primarily occurs through ingestion of milk/colostrum or inhalation of nasal secretions.3, 5 In utero transmission can occur infrequently in sheep and SRLVs have been detected in semen, though transmission via this route has not been documented.2, 3 In the present case, the dam of the affected goat kid tested positive for CAEV prior to pregnancy and the kid likely became infected following ingestion of colostrum and milk. This is further supported by the reported similar clinical signs in the sibling of the present animal.
Infection with SRLVs leads to slowly progressing, often subclinical, inflammatory disease.3 When clinical, four pathologic presentations, either alone or in combination, are recognized: encephalomyelitis, interstitial pneumonia, arthritis, indurative mastitis. Which form is present is variable and depends on various factors including species affected and age of the animal. Adult sheep typically present with pneumonia and/or encephalomyelitis while adult goats present with the arthritic form and goat kids present with the neurologic form.4, 5, 8
As in the present case, the neurologic form in both sheep and goat kids is histologically characterized by lymphocytic and/or histiocytic demyelinating leukoencephalomyelitis frequently resulting clinically in progressive ataxia beginning in the hindlimbs.3 The respiratory form is histologically characterized by lymphohistiocytic interstitial pneumonia, lymphoid follicle proliferation, and alveolar septa that are thickened by interstitial fibrosis and smooth muscle hypertrophy.3, 8 In sheep, type II pneumocyte hyperplasia is uncommon, while in goats it is frequently present.3 Additionally alveoli can be filled with abundant dense eosinophilic proteinaceous material.3, 8 Electron micrographs of this fluid reveal numerous myelin figures consistent with surfactant.9 In human medical literature, this accumulation of surfactant is termed alveolar proteinosis and is considered secondary to functional disruptions of alveolar macrophages.10 There are a variety of documented causes of alveolar proteinosis in humans while in goats it has been described with CAEV as well as pulmonary adenomatosis.9, 10 Therefore, alveolar proteinosis does not appear to be specific to CAEV but likely manifests secondary to alveolar macrophage dysfunction during infection.
The arthritic form, common in adult goats, is characterized by synovial villous hyperplasia with necrosis, mineralization, and fibrosis of the synovium with chronic infection.3 Finally, the mastitis form is frequently subclinical and is a significant source of economic losses. It is frequently non-painful and involves infiltration of the mammary interstitium by lymphocytes, plasma cells, and macrophages that with time progresses to fibrosis.3,5
The concurrent suppurative bronchopneumonia in this case is not typical for reported SRLV respiratory lesions. We suspect that this may represents a separate bacterial infection; multiple bacterial agents were cultured from the lung to support this hypothesis, but the weak growth of mixed bacteria makes it difficult to interpret whether this reflect true infection or contaminants. Mycoplasma spp. infection was also considered as an additional contributor, but PCR for this agent was negative.
Contributing Institution:
The Ohio State University College of Veterinary Medicine
Department of Veterinary Biosciences
Anatomic Pathology Service
1925 Coffey Road
Columbus, OH 43210
https://vet.osu.edu/departments-offices/biosciences
JPC Diagnosis:
- Lung: Pneumonia, interstitial, lymphohistiocytic, chronic, diffuse, marked, with peribronchiolar and perivascular lymphoid hyperplasia, type II pneumocyte hyperplasia, and alveolar proteinosis.
2. Lung: Bronchopneumonia, suppurative, subacute, multifocal, moderate.
3. Brainstem: Rhombencephalitis, necrotizing and lymphohistiocytic, subacute, focally extensive, severe, with gliosis.
JPC Comment:
The final case of this conference was descriptively rewarding for participants. The presence of brain and lung on the same slide from a young animal prompted many to immediately favor small ruminant lentiviruses, though there were several features of this case that were perplexing given the age of this animal. Foremost, the degree of alveolar proteinosis is marked, reflecting significant dysfunction of pulmonary macrophages. Given that these viruses utilize monocyte precursors both as a reservoir and means of trafficking virus to naïve tissue macrophages,11 we considered whether this might reflect coinfection (CAEV and OPP) and/or a distinct viral subtype that is more virulent. In theory, only few monocyte precursors retain lentivirus (i.e. susceptible but not permissive to viral replication) and it should take time to develop histiocytic interstitial pneumonia.
As the contributor notes, pneumonia is a more common finding in older animals (reflecting this notion) and is rarer in goats. It is likely that the goat kids in this case were secondarily infected with another agent that augmented monocyte recruitment and subsequent tissue macrophage activation and cytokine production that hampered surfactant handling.11 Notably, there is BALT hyperplasia present which is suggestive of Mycoplasma infection (or at least marked antigen presentation) which aligns with bacterial culture results and the airway-specific histological changes. For his reason, we separated this secondary bronchopneumonia out as a distinct morphologic diagnosis.
Dr. Highland concluded the conference discussion reminding participants about challenges in testing for small ruminant lentiviruses and emphasized that young animals with few infected monocytes may be seronegative or below detection limits of given assays. In larger goat herds and sheep flocks, the cost of serial testing presents a significant economic roadblock for controlling or eradicating these diseases.
References:
- Blacklaws BA. Small ruminant lentiviruses: immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus. Comp Immunol Microbiol Infect Dis. 2012 May;35(3):259-69.
- Blacklaws BA, Berriatua E, Torsteinsdottir S, et al. Transmission of small ruminant lentiviruses. Vet Microbiol. 2004 Jul 14;101(3):199-208.
- Caswell JL, Williams KJ. Respiratory system. In: Jubb, Kennedy and Palmer's Pathology of domestic animals, vol. 2. Sixth Edition. Elsevier. St.Louis, Mo, 2016:465-591.
- Cantile C, Youssef S. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, Palmer's Pathology of Domestic Animals. Vol.1. 6th ed. Philadelphia, PA: Elsevier; 2016:378-379.
- Gomez-Lucia E, Barquero N, Domenech A. Maedi-Visna virus: current perspectives. Vet Med (Auckl). 2018 May 21;9:11-21.
- Larruskain A, Jugo BM. Retroviral infections in sheep and goats: small ruminant lentiviruses and host interaction. Viruses. 2013 Aug 19;5(8):2043-61.
- Leroux C, Cruz JC, Mornex JF. SRLVs: a genetic continuum of lentiviral species in sheep and goats with cumulative evidence of cross species transmission. Curr HIV Res. 2010 Jan;8(1):94-100.
- Moroz A, Czopowicz M, Sobczak-Filipiak M, et al. The prevalence of histopathological features of pneumonia in goats with symptomatic caprine arthritis-encephalitis. Pathogens. 2022;11(6):629.
- Sims LD, Hale CJ, McCormick BM. Progressive interstitial pneumonia in goats. Australian Veterinary Journal. 1983;60:368-371.
- Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nature Reviews Disease Primers. 2019;5(1):16.
- Stanton JB, Zachary JF. Mechanisms of Microbial Infections. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. St. Louis, MO: Elsevier; 2022:254-255.