Signalment:
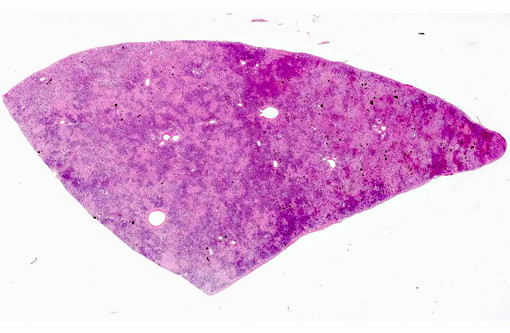
Gross Description:
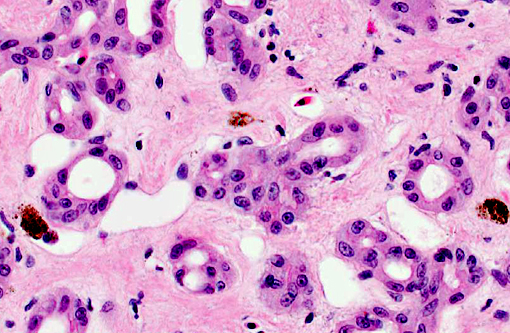
Histopathologic Description:
Morphologic Diagnosis:
1) Liver: Severe, diffuse, chronic, pseudocarcinomatous biliary hyperplasia with marked interstitial fibrosis.Â
2) Liver: Moderate biliary, hepatocellular and histiocytic iron accumulation.
Condition:
Contributor Comment:
Cholangiocarcinomas can have a massive or multilobular appearance, are often umbilicated and protrude from the liver capsule. Clinically, prolonged survival after initial diagnosis is also supportive of a diagnosis of PBH.Â
Pseudocarcinomatous biliary hyperplasia must also be differentiated from biliary hamartoma and cholangioma. In human and veterinary medicine, biliary hamartomas are rare and consist of ducts of varying caliber, unique cystic cavity formation and fibrosis.(15,17) In domestic animals, cholangiomas are usually solitary, well demarcated, round, cystic and solid masses, which grow by expansion and tend to bulge beyond the normal liver surface. In one retrospective study, 31% of all primary neoplasms in lizards affected the liver versus other organ systems,(16) with malignant biliary processes being the most frequent.
In veterinary medicine, biliary hyperplasia has been described in association with internal papillomatosis and chronic mycotoxicosis in avian species. Aflatoxins are excreted in the bile, causing periportal necrosis and inflammation in acute cases. With chronic exposure, there is bile duct hyperplasia and fibrosis, as described with chronic active hepatitis.(13) Severe biliary hyperplasia has been reported in an alpaca in association with parasitic ova of Fasciola hepatica,(8,14) and hepatic coccidiosis.(14) Experimental bile duct ligation or administration of alpha-naphthylisothiocyanate (ANIT), both producing obstructive cholestasis, or intravenous estradiol glucuronide, which causes non-obstructive cholestasis, have been shown to cause biliary epithelial cell hyperplasia in male Sprague-Dawley rats.(10)
In human medicine, the term pseudocarcinomatous hyperplasia is used to refer to marked epidermal proliferation with down growth into the dermis, associated with chronic granulomatous conditions of the underlying dermis or with keratinocyte atypic.(9) It has been described as hyperplastic glandular or ductular epithelium with a cribriform pattern, mimicking carcinoma, in different neoplastic and inflammatory processes. These include granular cell tumors,(2) anaplastic large cell lymphoma,(12) chronic osteomyelitis of the jaw and limbs,(18) oral syphilis infection,(1) and chronic salpingitis.(3) Pseudocarcinomatous urothelial hyperplasia of the urinary bladder has been associated with prior irradiation or chemotherapy and, recently, in several cases without such predisposing treatment.(11) Hyperplasia of biliary ducts in humans has been associated with biliary atresia, where the pathological changes include hyperplasia of canaliculi, inflammation, cholestasis and interstitial fibrosis of portal zones.(20)
The pathogenesis of pseudocarcinomatous hyperplasia (of the skin) has been related to epidermal growth factor (EGF) and transforming growth factor (TGF) elaborated by the primary tumor (e.g. lymphoma) or inflammatory cells.(4) Both of these growth factors have the same specific membrane receptor (EGFr) which has tyrosine kinase activity. The activation of this receptor can be involved in epithelial hyperplasia, wound healing and tumorigenesis.(7)
JPC Diagnosis:
Conference Comment:
Biliary hyperplasia is a nonspecific response to a variety of liver insults,(5) many of which are mentioned by the contributor. It is typically regarded as a result of long-standing hepatic injury, particularly after diseases which result in the obstruction of normal bile drainage.(5) It may also develop secondary to portal inflammation and fibrosis.(14) Ductular reaction is a term utilized when the progenitor cells with potential to differentiate into either biliary epithelium or hepatocytes proliferate, as also may occur in severe hepatic injury.(5) See the conference comments from WSC 2011-12, conference 3, case 2 for a detailed discussion of ductular reaction and its pathogenesis.
Diffuse hepatic fibrosis also corresponds with repeated toxic hepatic injury; however, this typically is followed by nodular regeneration as observed in a cirrhotic liver. When a single event induces widespread hepatocellular necrosis, fibrosis and condensation of preexisting connective tissue often occurs in the absence of regeneration and is termed postnecrotic scarring.(5) This is because the normal reticulin network of type III collagen, hepatic stellate cells, and nerves which occupy the space of Disse collapses, allowing portal triads to converge, giving rise to irregular bands of scar tissue.(14) Postnecrotic fibrosis, which develops around hepatic venules, is termed periacinar fibrosis and occurs commonly in cases of chronic passive congestion or pyrrolizidine alkaloid toxicity.(14)
In this case, the gross description and histopathologic findings best correlate with a diffuse, chronic hepatic insult. The presence of ascites is consistent with two previously reported cases,(18) and it would be interesting to compare clinical pathologic findings in this case to those previously reported to assist in determining whether the abdominal fluid is related to the hepatic lesion.
References:
1. Barrett AW, Dorrego MV, Hodgson TA, Porter SR, Hopper C, Argiriadou AS, Speight PM: The Histopathology of Syphilis of the Oral Mucosa. J Oral Pathol Med 33:286-291, 2004
2. Brannon RB, Anand PM: Oral Granular Cell Tumors: An Analysis of 10 New Pediatric and Adolescent Cases and a Review of the Literature. J Clin Pediatr Dent 29(1):69-74, 2004
3. Cheung ANY, Young RH, Scully RE: Pseudocarcinomatous Hyperplasia of the Fallopian Tube Associated with Salpingitis. Am J Surg Pathol 8(11):1125-1130, 1994
4. Courville P, Wechsler J, Thomine E, Vergier B, Fonck Y, Souteyrand P, Beylot-Barry M, Bagot M, Joly P, and The French Study Group On Cutaneous Lymphoma: Brit J Dermatol 140:421-426, 1999
5. Cullen JM, Brown DL. Hepatobiliary system and exocrine pancreas. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:415-418.
6. Cullen JM, Popp JA: Tumors of the Liver and Gall Bladder. In: Tumors in Domestic Animals, ed Meuten DJ, 4th ed., pp 483-508. Blackwell Publishing, Ames IA 2002
7. De Boer WI, Houtsmuller AB, Izadifar V, Muscatelli-Groux B, Van Der Kwast TH, Chopin DK: Expression and Functions of EGF, FGF and TGF beta-Growth-Factor Family Members and Their Receptors In Invasive Human Transitional Cell Carcinoma Cells. Int J Cancer 71:284-291, 1997
8. Hamir AN, Smith BB: Severe Biliary Hyperplasia Associated with Liver Fluke Infection in an Adult Alpaca. Vet Pathol 39:592-594, 2002
9. KawachiY, Taguchi S, Fijisawa Y, Furuta J, Nakamura Y, Ishii Y, Takahashi T, Otsuka F: Epidermal Pseudocarcinomatous Hyperplasia With Underlying Epidermal Growth Factor-Producing Cutaneous CD30-Positive Lymphoproliferative Disorder. J Eur Acad Derm Venereology, Letter to the Editor, 2008
10. Kossor DC, Meunier PC, Dulik DM, Leonard TB, Goldstein RS: Bile Duct Obstruction Is Not A Prerequisite for Type I Biliary Epithelial Cell Hyperplasia. Tox and Appl Pharmacol152:327-338, 1998
11. Lane Z, Epstein JI: Pseudocarcinomatous Epithelial Hyperplasia in the Bladder Unassociated With Prior Irradiation or Chemotherapy.Am J Surg Path 32:92-97, 2008
12. Lin J, Lee JY: Primary Cutaneous CD30+ Anaplastic Large Cell Lymphoma With Keratoacanthoma-Like Pseudocarcinomatous Hyperplasia and Marked Eosinophilia and Neutrophilia. J Cutan Pathol 32:458-461, 2004
13. Schmidt RE, Reavill DR, Phalen DN: Liver. In: Pathology of Pet and Aviary Birds, 1st ed., pp 67-93. Blackwell Publishing, Ames, IA, 2003
14. Stalker MJ, Hayes MA: Liver and Biliary System. In: Pathology of Domestic Animals, ed Maxie MG, 5th ed., pp 298-387. Elsevier Saunders, Philadelphia, PA, 2007
15. Starost MF: Solitary Biliary Hamartoma with Cholelithiasis in a Domestic Rabbit (Oryctolagus cuniculus). Vet Pathol 44:92-95, 2007
16. Sykes IV JM, Trupkiewicz JG: Reptile Neoplasia at the Philadelphia Zoological Garden 1901-2002. J Zoo Wildlife Med 37(1):11-19, 2006
17. Tohm+�-�-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V: Multiple Biliary Hamartomas: Magnetic Resonance Features With Histopathologic Correlation. Eur Radiol 18:493-499, 2008
18. Warter A, Walter P, Meyer C, Barri+�-�re P, Galatir L, Wilk A: Mandibular Pseudocarcinomatous Hyperplasia. Histopathology 37:115-117, 2000
19. Wilson GH, Fontenot DK, Brown CA, Kling MA, Stedman N, Greenacre CB: Pseudocarcinomatous Biliary Hyperplasia in Two Green Iguanas, Iguana iguana. J Herpetological Med & Surg 14(4):12-18, 2004
20. Zheng S, Luo Y, Xiao X: Analysis of the Pathomorphology of the Intra- and Extrahepatic Biliary System in Biliary Atresia. Eur J Pediatr Surg 18:98-102, 2008