Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 15
16 January 2019
CASE I: 16153 E (JPC 4102152).
Signalment: 7 year old, male, rhesus macaque (Macaca mulatta)
History: This animal received total body irradiation (4 Gy) as part of an experimental protocol, and demonstrated persistent pyrexia (104 °-105 ° F), inappetence, weight loss and was persistently pancytopenic despite aggressive supportive care, blood transfusions and antibiotic therapy (Enrofloxacin, Ceftiofur and Ertepenem). The animal was euthanized 47 days post-irradiation due to poor prognosis and non-response to treatment.
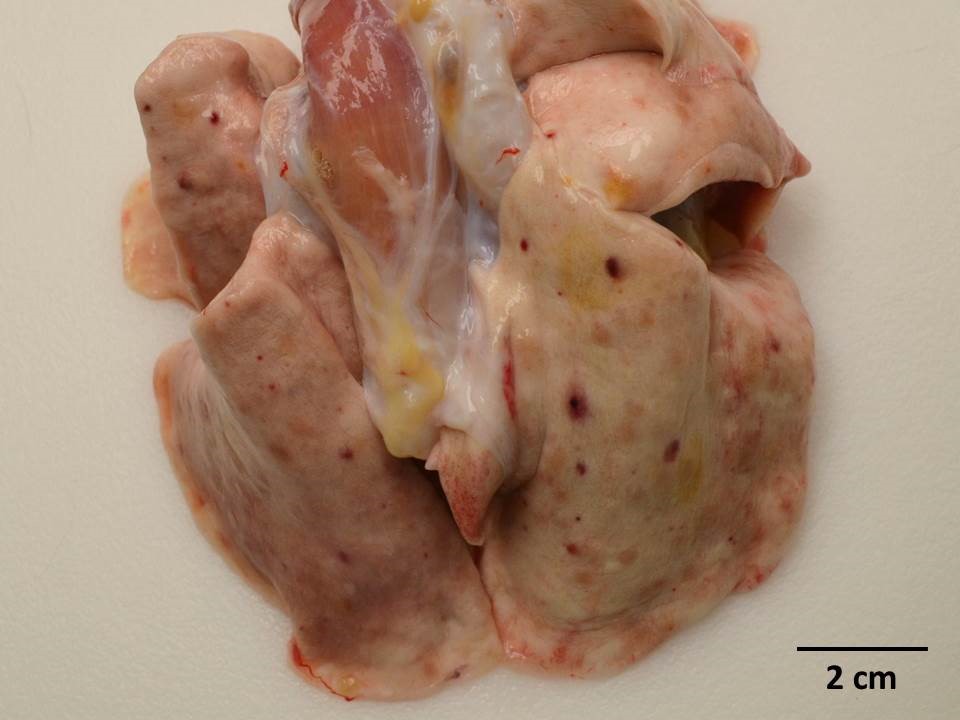
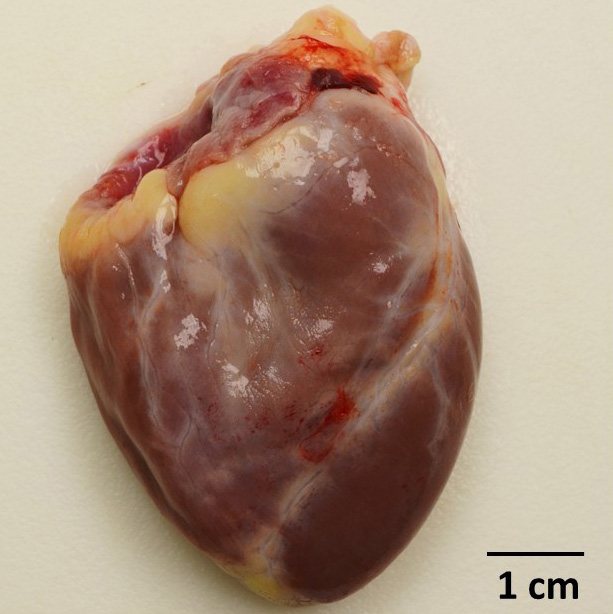
Gross Pathology: Dozens of petechiae, ecchymoses and pinpoint to 1.4 mm diameter, soft, yellow-tan foci and were scattered throughout the parenchyma of both lungs. Additional petechiae and ecchymoses were scattered throughout the left lateral lobe of the liver and urinary bladder. The epicardium of the right ventricle was irregularly thickened by off-white opaque material.
Gross Pathology: Dozens of petechiae, ecchymoses and pinpoint to 1.4 mm diameter, soft, yellow-tan foci and were scattered throughout the parenchyma of both lungs. Additional petechiae and ecchymoses were scattered throughout the left lateral lobe of the liver and urinary bladder. The epicardium of the right ventricle was irregularly thickened by off-white opaque material.
Laboratory results: Blood culture: No growth
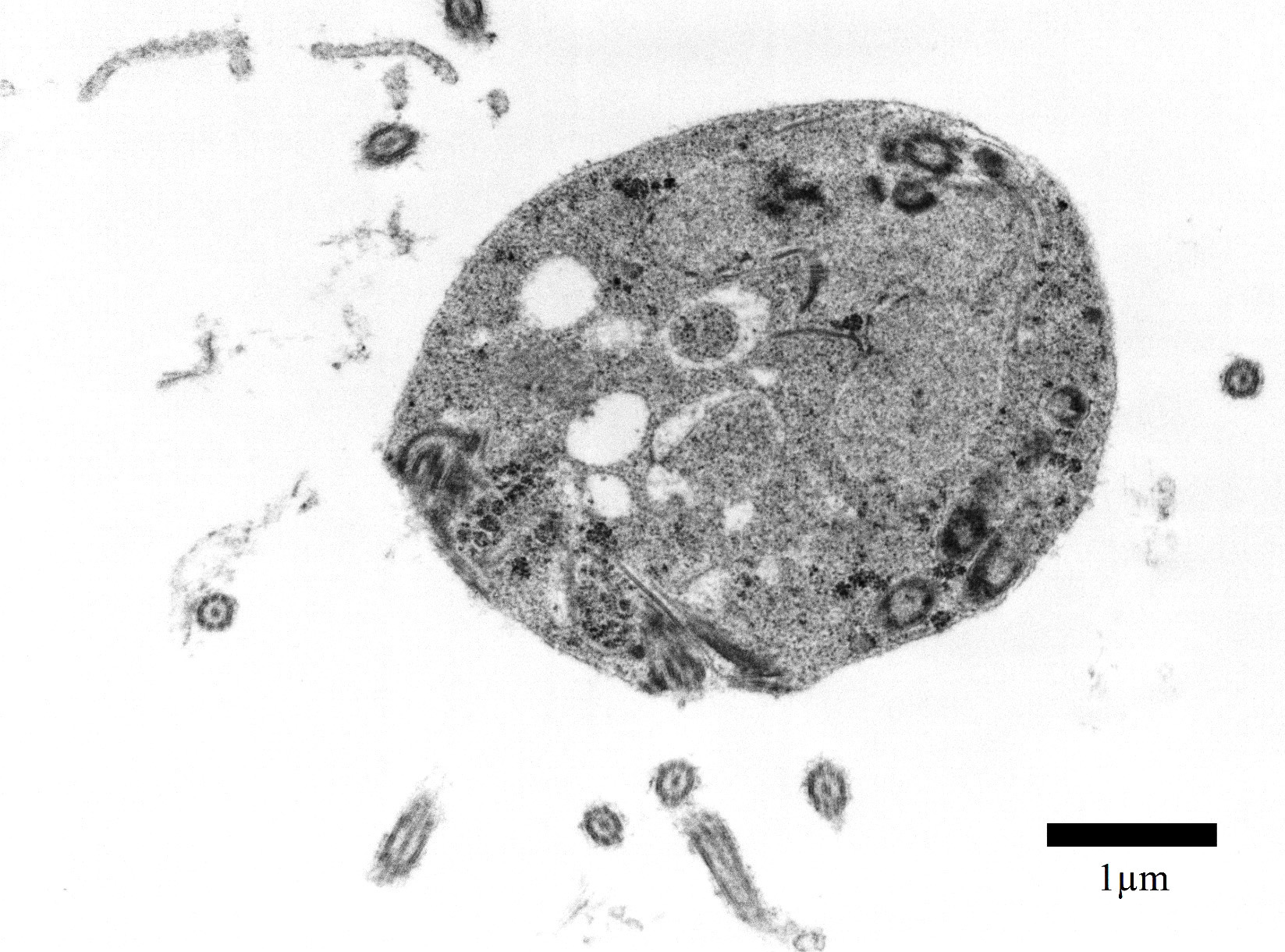
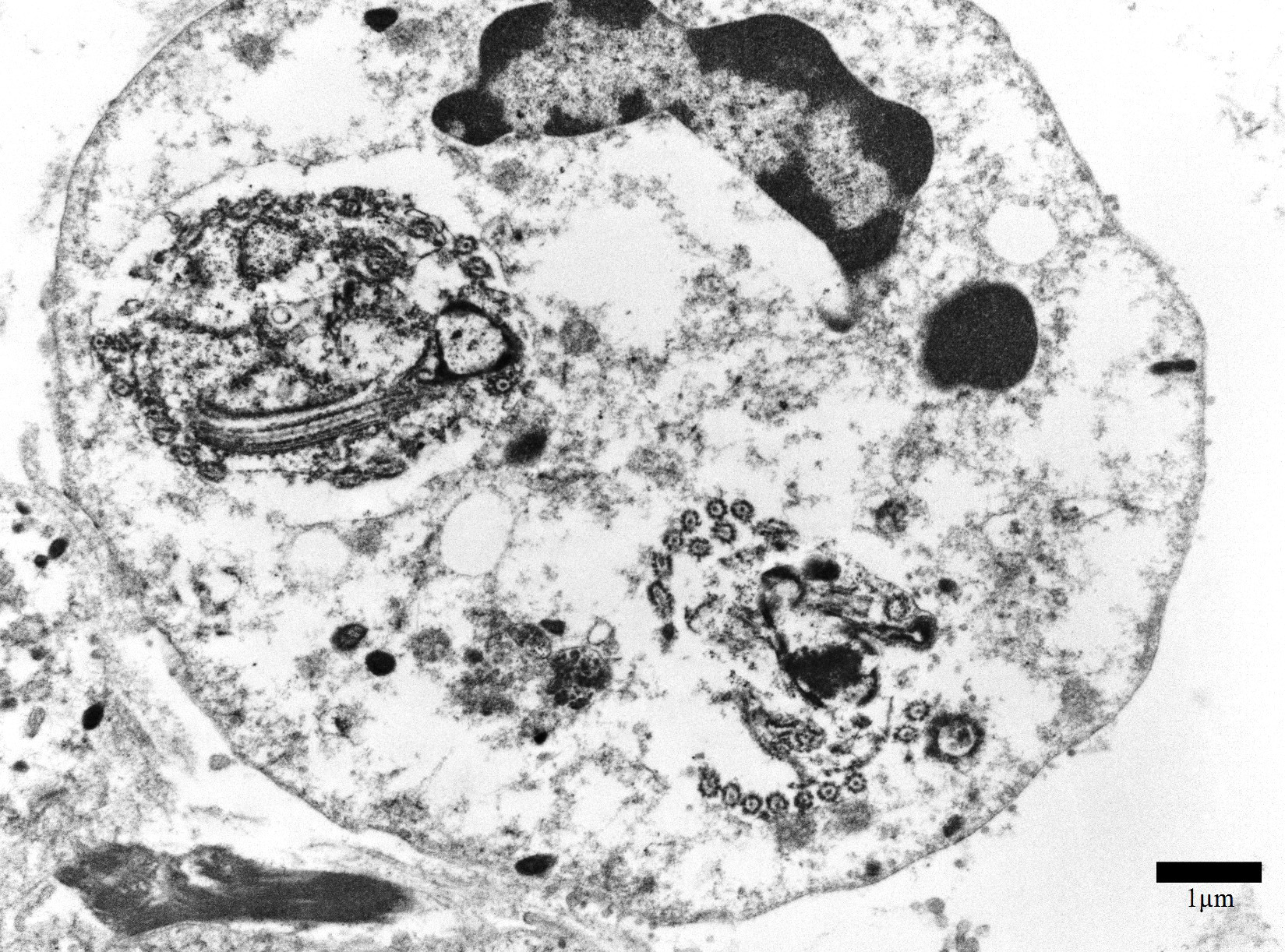
Transmission electron microscopy, epicardial adipose tissue: There were numerous 3-4μm diameter extracellular and intrahistiocytic protozoa with cilia projecting from the lateral margins, often arranged in parallel rows, moderately electron dense grainy cytoplasm, two electron dense nuclei, and a single internal marginated flagellum.
Microscopic Description:
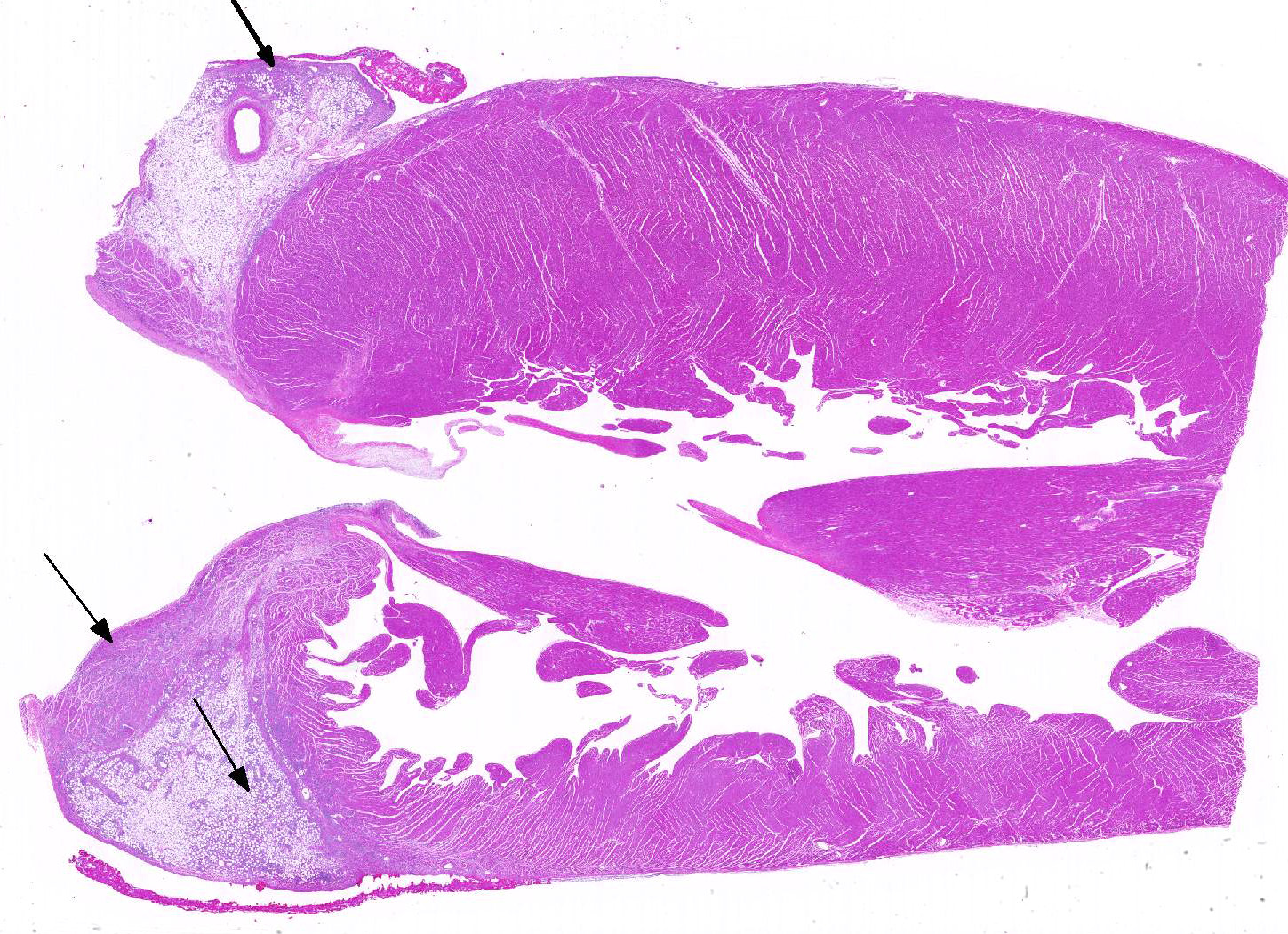
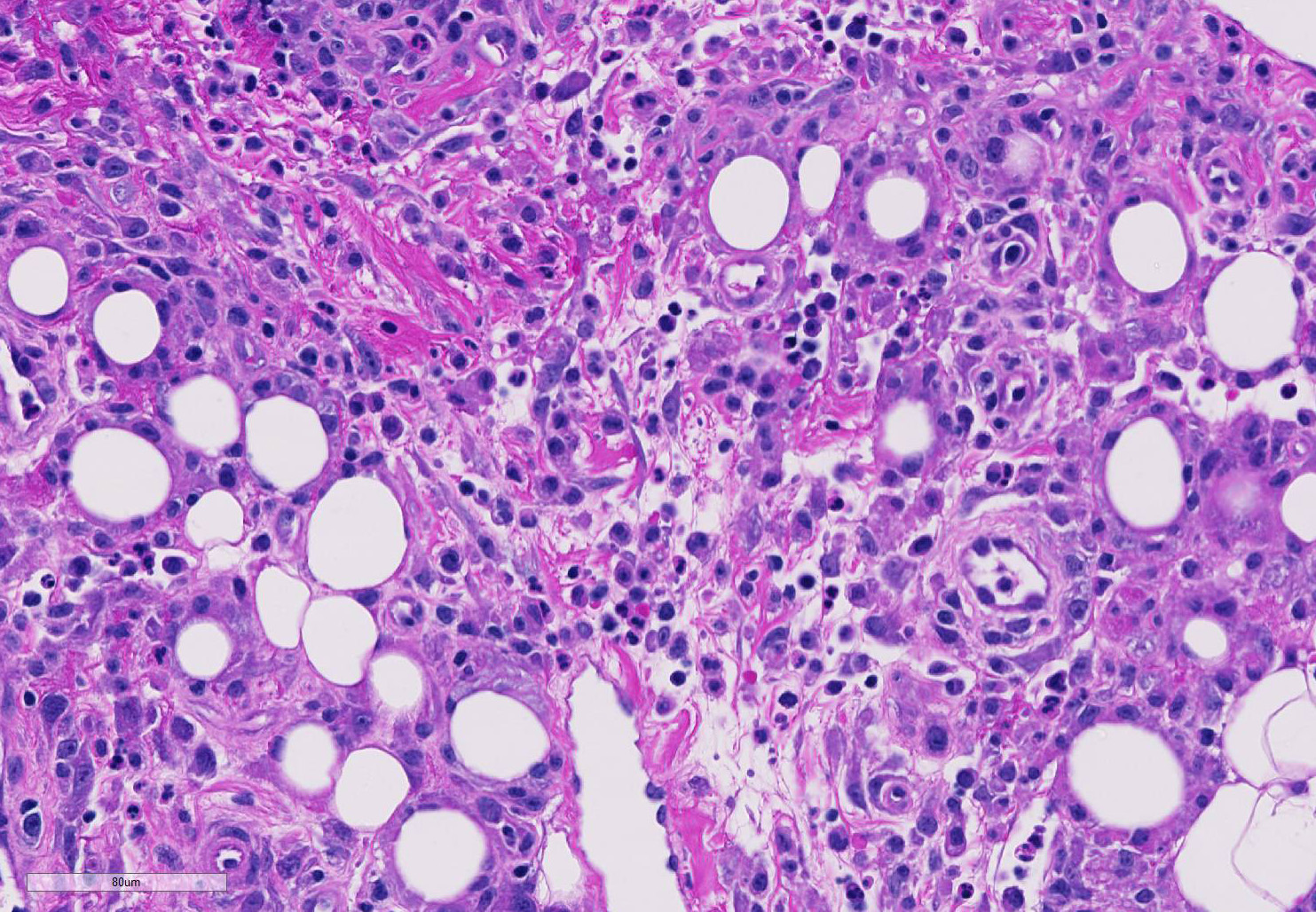
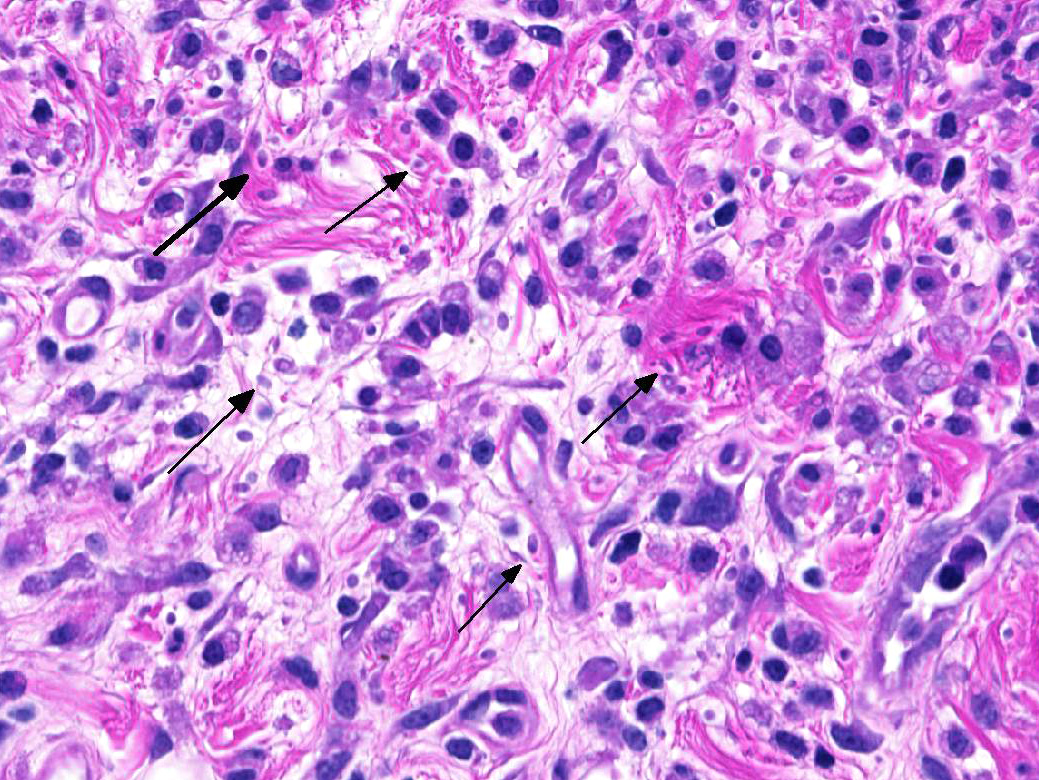
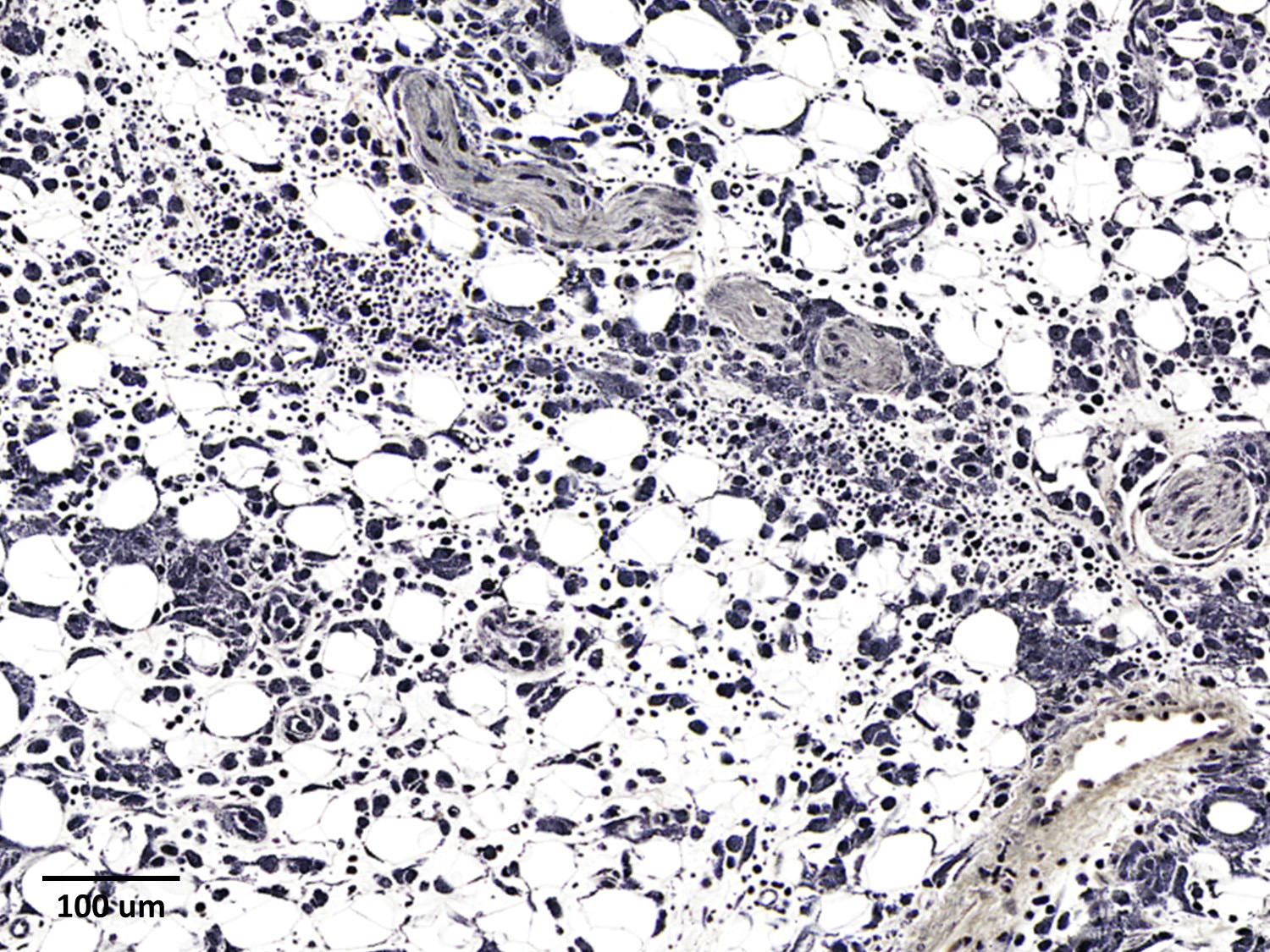
The epicardium and epicardial adipose tissue is infiltrated by coalescing aggregates of lymphocytes, plasma cells, macrophages, and scattered foci of neutrophils, all of which dissect between and separate adipocytes, and extend into the underlying myocardium. Innumerable, free and intrahistiocytic, 3-4µm diameter, oval, eosinophilic to amphophilic protozoa are scattered throughout the affected tissue. The protozoa are surrounded by a 10µm diameter clear space within the matrix. The protozoa stain positively with Heidenhain iron hematoxylin (HIH); and are negative for periodic acid-Schiff (PAS), Groncott-Gomori methenamine silver stain (GMS), and mucicarmine.
The epicardial surface is overlain by sheets of loose, fibrillar eosinophilic material (fibrin), containing scattered lymphocytes and histiocytes.
Contributor’s Morphologic Diagnoses:
Epicarditis, pericarditis, myocarditis, and epicardial steatitis, regionally extensive, chronic, severe, lymphohistiocytic with intralesional protozoa
Contributor’s Comment: Spironucleus species are flagellated diplomonad protozoans which are found as gastrointestinal (GI) commensals and may also act as opportunistic parasites in an array of hosts including birds, fish, and mammals. Generally, the parasites live within the intestinal lumen or crypts, with clinical signs in infected animals either not observed or limited to GI-related symptoms including diarrhea, anorexia, and wasting4,11. Gross pathologic changes depend on the severity of disease, and may include gas-distended intestines, enteritis, and ascites.3
While Spironucleus species rarely cause extra-intestinal disease, such incidents have been previously documented in Atlantic salmon12, Atlantic char13, Siamese fighting fish, cichlids10, and rhesus macaques2. Immunocompromised animals have a greater risk of developing an overwhelming infection; for example, athymic mice infected with Spironucleus have increased mortality.4 Reported pathogenic infections in macaques have been in animals infected with Simian Immunodeficiency virus.2,8
This animal was one of three in a cohort of animals that had received experimental whole body irradiation. All three animals had systemic Spironucleus infection where protozoa were positively identified and confirmed by PCR. Speciation is pending. Lesions included polyserositis, epicarditis, ureteritis, cystitis, epididymitis, and pneumonia. One animal had protozoa in the cerebrospinal fluid.
Spironucleus, formerly classed as Hexamita or Octomitus in some species9,11, may be difficult to detect on routine histologic sections. Histologic stains used to identify them are hematoxylin and eosin (H&E), Giemsa, Gomori’s methenamine silver (GMS), and Heidenhain iron hematoxylin (HIH).14 The protozoa from the three cases from our institution appeared amphophilic on H&E and Giemsa, were negative for GMS, and prominently stained deep purple with HIH. Other previously utilized techniques for diagnosis include direct smear of intestinal contents, immunochemical techniques on feces, and polymerase chain reaction.5,6. Electron microscopy (EM) has also been used as a tool to positively identify Spironucleus species, which, on TEM depending on speciation, range from 2-4x4-20µm have lateral cilia and a recurrent flagellum which arises from an indent in the nuclear membrane near two nuclei, which form an ‘S’-shape.2,12
Contributing Institution:
Wake Forest School of Medicine
Department of Pathology, Section on Comparative Medicine
Medical Center Boulevard
Winston-Salem, NC 27157
www.wakehealth.edu
JPC Diagnosis: Heart: Epicarditis and steatitis, histiocytic and neutrophilic, multifocal to coalescing, marked with mild myocarditis and endocarditis and numerous intrahistiocytic and extracellular protozoa.
JPC Comment: The contributor has provided an excellent discussion of this rarely reported systemic condition in immunosuppressed macaques. A very similar case of systemic spironucleosis was included in the 2011-2012 Wednesday Slide Conference (Conference 19, case 3) although the submitted tissue was colon.8
Reports of Spironucleus in non-human primates are rare in both health and disease. To date, the rhesus macaque appears to be the only species of non-human primate in which this genus (specifically, S. pitheci) has been identified2, and all other reports of Spironucleus (largely that as a gut commensal) are in laboratory rodent species. Immunosuppression in rodent species may also lead to systemic infection.
Spironucleus and other related flagellates such as Giardia and Entamoeba are interesting in their evolutionary development of a highly reductive organelle to take the place of mitochondria, known as a “mitosome” (or in the case of Spironucleus, the “hydrogenosome”).1 These organelles, like mitochondria, have a double membrane, and are associated with a number of proteins closely related to those found in mitochondria, Unlike mitochondria, mitosomes do not contain their own genes; genes for their genesis and function are present in the nuclear genome. Mitosomes do not generate ATP through the process of oxidative phosphorylation similar to other eukaryotes. Energy generation in these species is largely a process of anaerobic glycolysis with the conversion of glucose to pyruvate occurring in the cytosol, and further oxidation of pyruvate occurring in the hydrogenosome.1
An extremely important component of this particular case is the immunosuppression required for systemic infection by the offending diplomonad. The hematopoietic syndrome is a well-recognized subsyndrome of acute radiation exposure (along with the gastrointestinal and cerebrovascular subsyndromes).6 Lymphocytes are extremely sensitive to ionizing radiation and rapidly disappear from the circulation following whole-body irradiation. This is followed by neutrophil loss and then finally platelet loss over the course of several days as existing populations are used up and not replaced by the damaged marrow.6 At this point, impaired immunity may result in a wide range of opportunistic infections, or the animal may succumb to fatal hemorrhage as a result of thrombocytopenia.6
References:
- Adam R. Biology of Giardia lamblia. Clin Microbiol Rev 2001; 14(3):447-445.
- Bailey C, Kramer J, Mejia A, MacKey A, Mansfield KG, and Miller AD. Systemic Spironucleosis In two immunodeficient rhesus macaques (Macaca mulatta). Vet Pathol. 2010;47(3):488–494.
- Baker D. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev. 1998;11:231-66.
- Boorman GA, Lina PHC, Zurcher C, and Nieuwerkerk HTM. Hexamita and Giardia as a cause of mortality in congenitally thymus-less mice. Clin Exp Immunol. 1973;15:623–627.
- Fain MA, Karjala Z, Perdue KA, Copeland MK, Cheng LI, and Elkins WR. Detection of Spironucleus muris in unpreserved mouse tissue and fecal samples by using a PCR assay. J Am Assoc Lab Anim Sci. 2008;47:39–43.
- Gulani J, Koch A, Chappell MG, Christensen CL, Facemire P, Singh V, Ossetrova NI, Srinivasan V, Holt RK. Cercopithecine herpesvirus-9 (Simian varicella virus) infection after total-body irradiation in a rhesus macaque. Comp Med 2016; 66(2):150-153
- Jackson GA, Livingston RS, Riley LK, Livingston BA, and Franklin CL. Development of a PCR assay for the detection of Spironucleus muris. J Am Assoc Lab Anim Sci. 2013;52(2):165-170.
- Joint Pathology Center Veterinary Pathology Services Wednesday Slide Conference: Conference: 19 - 2011 Case: 03 Colon – Macaque. c2011. Cited 2017 June 5. Available from: https://www.askjpc.org/wsco/wsc_showconference.php?id=492
- Jørgensen A, Sterud E. Phylogeny of Spironucleus (Eopharyngia: Diplomonadida: Hexamitinae) Protist. 2007;158:247–254.
- Paull GC MR: Spironucleus vortens, a possible cause of hole-in-the-head disease in cichlids. Diseases of Aquatic Organisms 2001;45:197-202.
- Poynton S. Diplomonad (Hexamitid) Flagellates: Diplomonadiasis, Hexamitosis, Spironucleosis. In: AFS-FHS (American Fisheries Society-Fish Health Section), FHS Blue Book: Suggested Procedures for the Detection and Identification of Certain Finfish and Shellfish Pathogens, 2007 ed., AFS-FHS, Bethesda, MD.
- Sterud E, Atle Mo T, Poppe TT. Systemic spironucleosis in sea-farmed Atlantic salmon Salmo salar, caused by Spironucleus barkhanus transmitted from feral Arctic char Salvelinus alpinus. Ds Aquat Org. 1998;33:63-66.
- Sterud E, Poppe TT, Bornø G. Intracellular infection with Spironucleus barkhanus (Diplomonadida, Hexamitidae) in farmed Arctic char Salvelinus alpinus. Dis Aquat Org. 2003;56:155–61.
- Wood AM, Smith VH. Spironucleosis (Heramitiasis, Hexamitosis) in the ring-necked pheasant (Phasianus colchicus): Detection of cysts and description of Spironucleus meleagridis in stained smears. Avian Dis. 2005;49:138–143.