Signalment:
Gross Description:
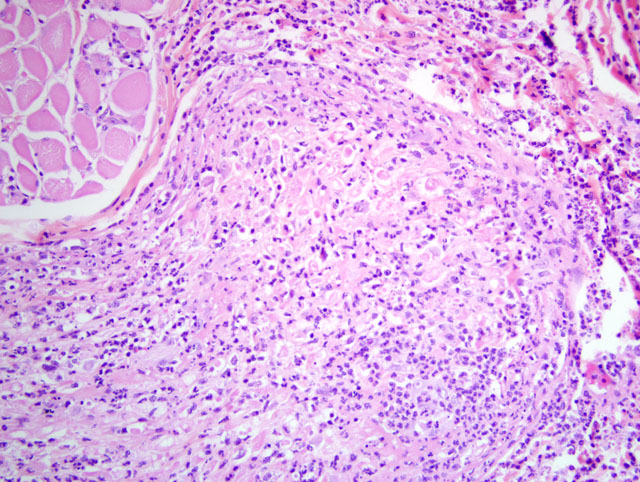
Histopathologic Description:
Morphologic Diagnosis:
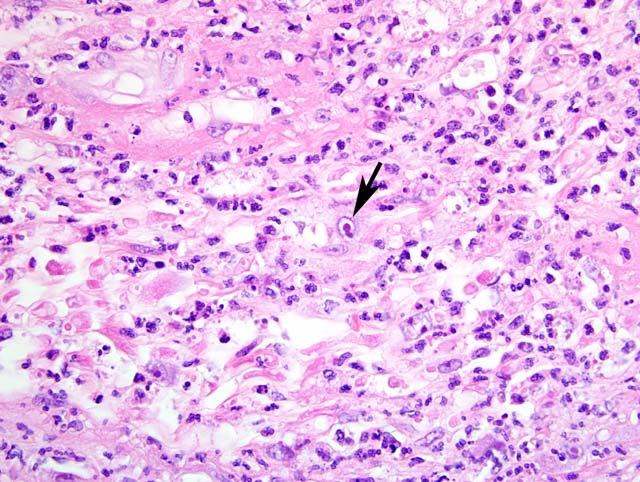
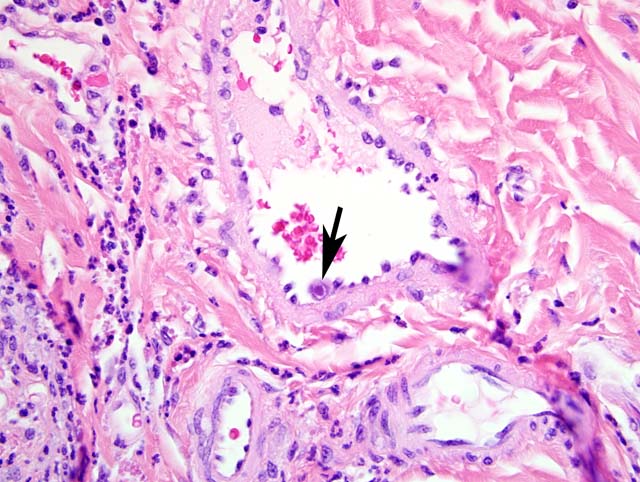
1. Labial mucosa and haired skin: Severe, multifocal, subacute neutrophilic neuritis with cytomegaly and intrahistiocytic, intranuclear and intracytoplasmic herpetic inclusions (Cowdry type A).
2. Labial mucosa and haired skin: Severe, multifocal, subacute to chronic neutrophilic folliculitis with perifollicular fibrosis, cytomegaly, and intrahistiocytic, intranuclear and intracytoplasmic herpetic inclusions (Cowdry type A).
Lab Results:
Condition:
Contributor Comment:
Common clinical and pathologic findings in SIV infected rhesus macaques include lymphadenopathy/lymphoma, chronic diarrhea and wasting, giant cell disease, pulmonary arteriopathy, viral associated dermatitis, and a wide spectrum of opportunistic infections. The most common opportunistic infectious agents in rhesus macaques progressing to AIDS include Pneumocystis carinii, Mycobacterium avium complex, Trichomonas sp., cytomegalovirus, adenovirus, Cryptosporidium, SV-40, Candida sp., and rhesus lymphocryptovirus.(10) Additionally, infections with Enterocytozoon bienusi, Entamoeba, Giardia and various alphaherpes viruses are also seen regularly.Â
Cytomegaloviruses (CMV) are host-specific beta-herpesviruses that frequently establish latent infections in both rhesus macaques and humans. In rhesus macaques, CMV seroprevalence approaches 100% by one year of age but there are generally no clinical or pathologic findings associated with infection in otherwise healthy individuals.(3,11) Transmission is thought to be through contact with virus shed in urine, saliva, and genital secretions which is similar to findings in humans. Unlike in humans, vertical transmission has not been documented, most likely due to near 100% seroprevalence with resultant high maternal antibodies and fetal protection.Â
With immune suppression, as occurs with AIDS, latent CMV becomes reactivated. It is the most common viral opportunistic infection identified and among the most common opportunistic infections overall in both rhesus macaques and man with isolation from the retina, gastrointestinal tract, lungs, and adrenal gland common in humans and gastrointestinal tract, lungs, central nervous system, liver and lymph nodes in macaques.(4,5)
Common gross pathologic features of cytomegalovirus infection depend on the organ involved. A common finding at the NEPRC is gastrointestinal pseudotumors which present as raised, red lesions multifocally throughout the small intestine.(7) Less common gross findings include multifocal interstitial pneumonia, necrotizing orchitis, and gastrointestinal ulceration. Histologically, lesions are frequently characterized by intense neutrophilic infiltrates admixed with fewer lymphocytes and plasma cells. Scattered throughout these areas there are frequently large intranuclear inclusion bodies, so-called owls eye cells and also smaller intracytoplasmic inclusion bodies.Â
Unfortunately, these cells are not always present and as many as one half of infected animals may show varying degrees of pathology without characteristic inclusion bodies making immunohistochemistry important for definitive diagnosis of suspected cases. In fact, immunohistochemistry has shown that some animals with no underlying pathology have viral reactivation in multiple organs.(5)
In this case, the CMV-associated neuritis and folliculitis of the labial mucosa is quite striking. While not a common finding with CMV reactivation, neuritis is seen occasionally in rhesus macaques at the NEPRC and is most commonly seen in the labial and buccal mucosa. In humans, CMV optic neuritis and retinitis is a common finding. We have not previously identified folliculitis and this is an uncommon finding in humans as well.Â
JPC Diagnosis:
1. Haired skin and mucocutaneous junction, lip: Polyneuritis, suppurative, acute, moderate, with axonal degeneration and intranuclear and intracytoplasmic eosinophilic and amphophilic inclusion bodies.
2. Haired skin and mucocutaneous junction, lip: Epidermal hyperplasia, focally extensive, moderate, with hyperkeratosis.
Conference Comment:
| Cytomegaloviruses in Veterinary Species | |||
| Name | Virus Subfamily | Species Affected | Summary |
| Simian CMV (includes Rhesus CMV) | Betaherpesvirus | Rhesus macaque; other NHP have host-specific CMV | Seroprevalence nearly 100% in rhesus macaques; clinical disease occurs only with immunosuppression (SIV) or experimental manipulation |
| Caviid herpesvirus | Betaherpesvirus | Guinea pig | Subclinical infection is common; useful model of human CMV; typical "owls eye" inclusions; targets are salivary gland, kidney, and liver |
| Murine CMV (Murid herpesvirus 1) | Betaherpesvirus | Mouse | BALB/c and A strain mice are susceptible; B6, B10, CBA, and C3H mice are resistant; disease occurs in immunocompromised mice; typical lesions are in salivary glands; persistent infections may cause immune complex glomerulitis |
| Rat CMV | Betaherpesvirus | Rat | Common in wild rats; nonexistent in laboratory rats; typical lesions in salivary and lacrimal glands with intranuclear and intracytoplasmic inclusions in ductal epithelium |
| Bovine herpesvirus 4 | Gammaherpesvirus | Ox | Associated with "epivag" (i.e. vaginitis, salpingitis, oophoritis, or epidiymitis), pneumonia, enteritis, metritis, mammillitis, and abortion |
| Suid herpesvirus 2 | Betaherpesvirus | Pig | Causes inclusion body rhinitis in neonates; infection in pregnant sows results in small litters, mummification, stillborith, or weak, premature offspring; typical cytomegaloviral inclusions in nasal mucosa, lung, or kidney, but not seen in the placenta |
| Equid herpesvirus 2 | Gammaherpesvirus | Horse | Clinical significance unknown; has been identified in few cases of bronchointerstitial pneumonia in foals |
The contributors observation of inflammation involving the hair follicles was also discussed. Most conference participants interpreted the folliculitis as a secondary extension of the neurocentric inflammation into the sinus hairs, rather than primary folliculitis due to viral infection of hair follicles. When present, inflammation of the hair follicles was generally confined to well-innervated sinus hairs, and in less severely affected follicles, the inflammatory cells appeared to extend from the peripheral nerve into the stroma surrounding the adnexa.Â
References:
2. Jellinger KA, Setinek U, Drlicek M, Bohm G, Steurer A, Lintner F: Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol 100 :213-220, 2000
3. Kuhn EM, Stolte N, Matz-Rensing K, Mach M, Stahl-Henning C, Hunsmann G, Kaup FJ: Immunohistochemical studies of productive rhesus cytomegalovirus infection in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol 36:51-56, 1999
    a. Geretti AM: Simian immunodeficiency virus as a model of human HIV disease. Rev Med Virol 9:57-67, 1999
    b. Caswell JL, Williams KJ: Respiratory system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, p. 569. Elsevier Saunders, Philadelphia, PA, 2007
4. McAdam AJ, Sharpe AH: Infectious diseases. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 353-355. Saunders Elsevier, Philadelphia, PA, 2010
5. Mohan H, Bal A, Garg S, Dalal U: Cytomegalovirus-associated pseudotumor simulating gastric malignancy in acquired immunodeficiency syndrome: a case report with review of literature. Jpn J Infect Dis 60:134-136, 2007
6. Percy DH, Barthold SW: Pathology of Laboratory Rodents and Rabbits, 3rd ed., pp. 19-21, 127, 221-222. Blackwell Publishing, Ames, IA, 2007
7. Schlafer DH, Miller RB: Female genital system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 528-529, 531-532. Elsevier Saunders, Philadelphia, PA, 2007
8. Simon MA, Chalifoux LV, Ringler DJ: Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses 8:327-337, 1992
9. Yue Y, Barry PA: Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv Virus Res 72:207-226, 2008