Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 16
16 January, 2019
CASE III: 11727 (JPC 4019850).
Signalment: 3-years-old, female, crossbreed, heifer (Bos taurus)
History: A herd of 70 heifers were in a wetland during the summer (since February 2010). These three-years-old heifers had been transferred to two years rice stubble, next to a facility to calve (August 2011). In December 2011, five animals died after 30-40 days of weight loss. The heifers had been treated with Nitroxynil in May and September 2011 before calving and with Ivermectin in October 2011 after calving. One heifer was recumbent for 12 hour and was euthanized due to unfavorable prognosis and was immediately necropsied.
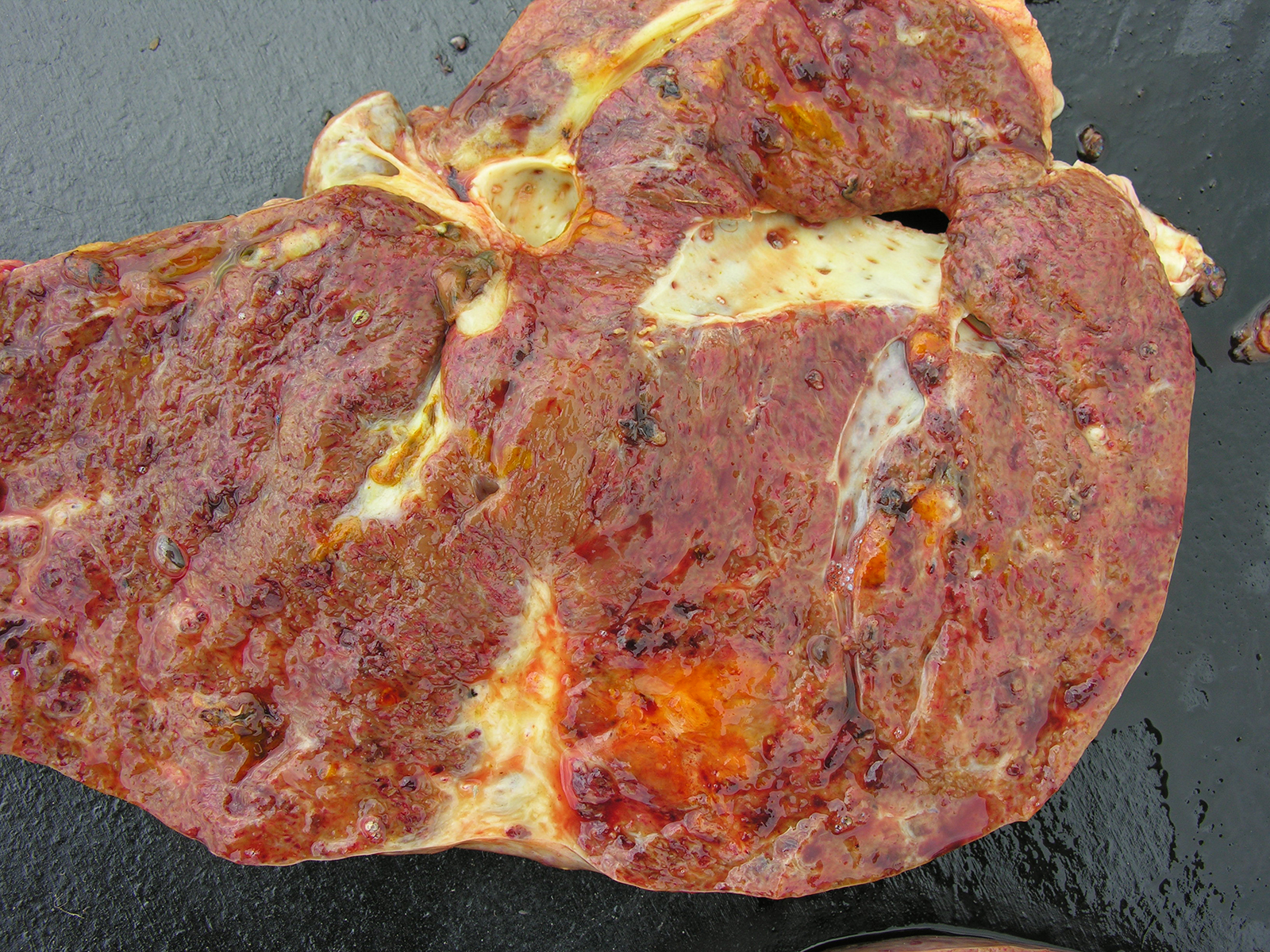
Gross Pathology: At necropsy, mucosae showed mild anemia and jaundice. Dark brown ascitic fluid was present in abdominal cavity and there were a thick layer of fibrin among peritoneum, intestines, diaphragm and liver. The liver was enlarged with rounded edges and the capsular surface was irregular with adhered fibrin and red strips interspersed with clear areas and petechiae. Liver cut surface was irregular, friable and hemorrhagic and white channels could be observed through the parenchyma. There were some dark foci filled with debris and adult forms of Fasciola hepatica. The gallbladder was enlarged and the wall was thick and edematous and contained some adult forms of F. hepatica. Hepatic lymph nodes were enlarged and wet and fluid was drained on the cut hemorrhagic surface. Renal infarction was also observed. Fibrin and clotted blood were observed adhered to the pericardium and lung, mainly in diaphragmatic lobes.
Laboratory results: no tests were performed
Microscopic Description:
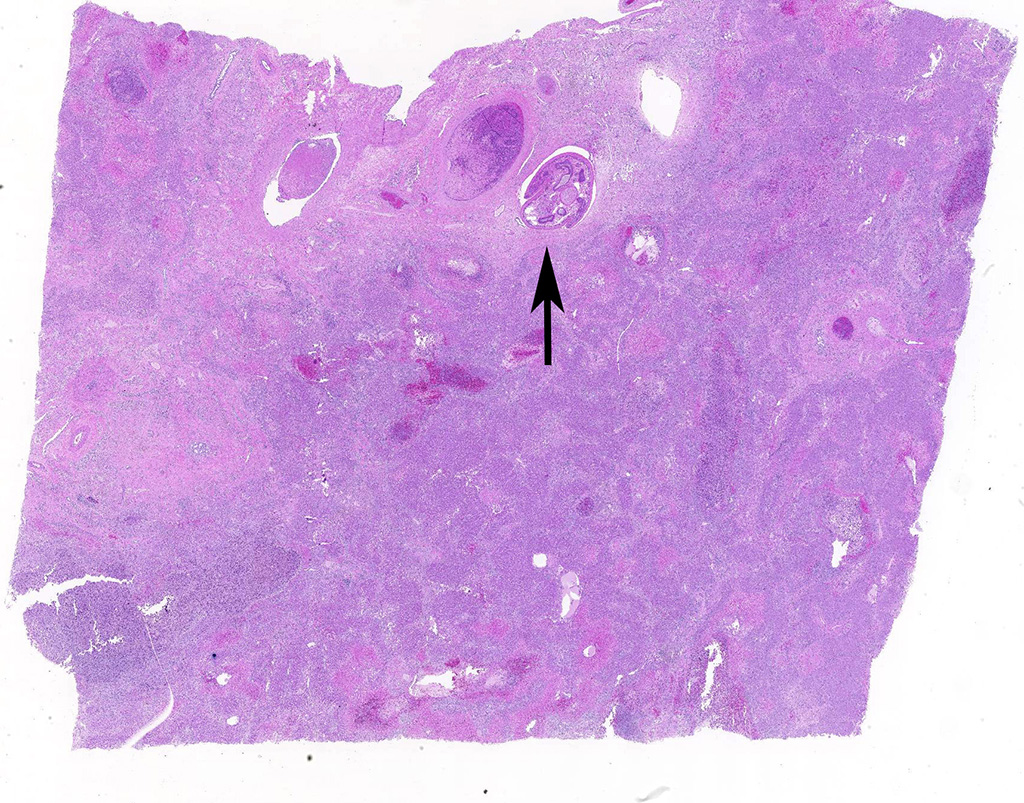
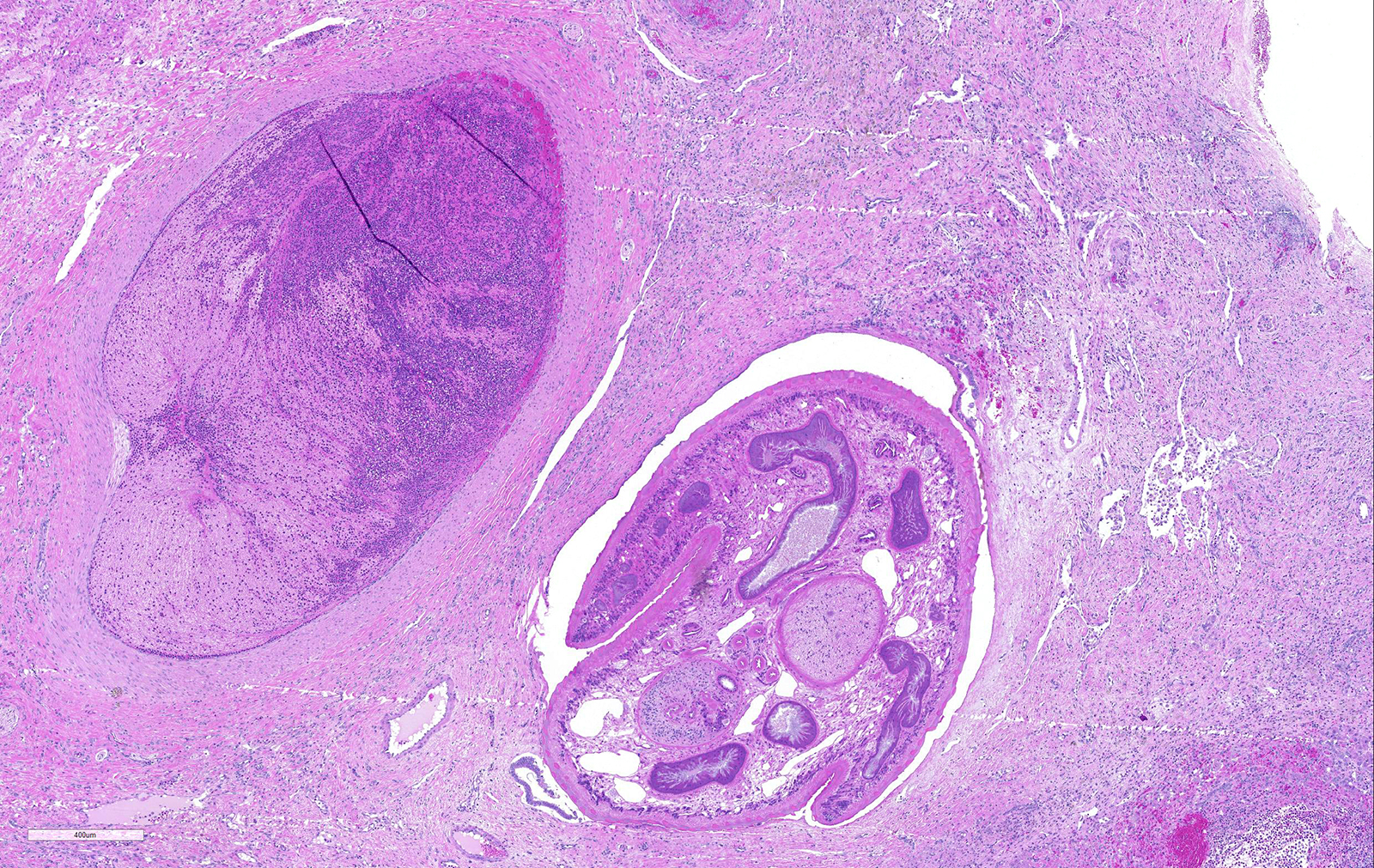
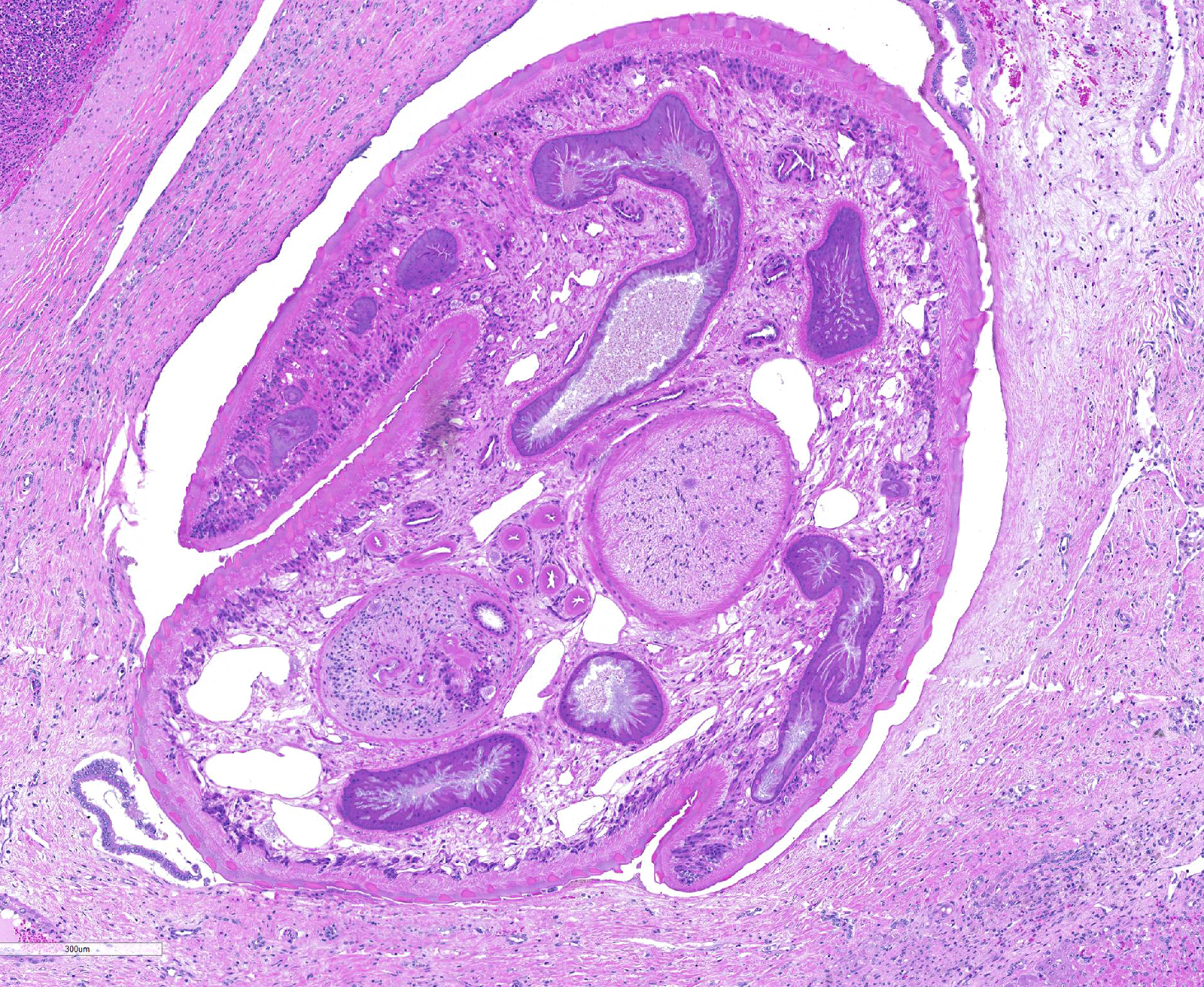
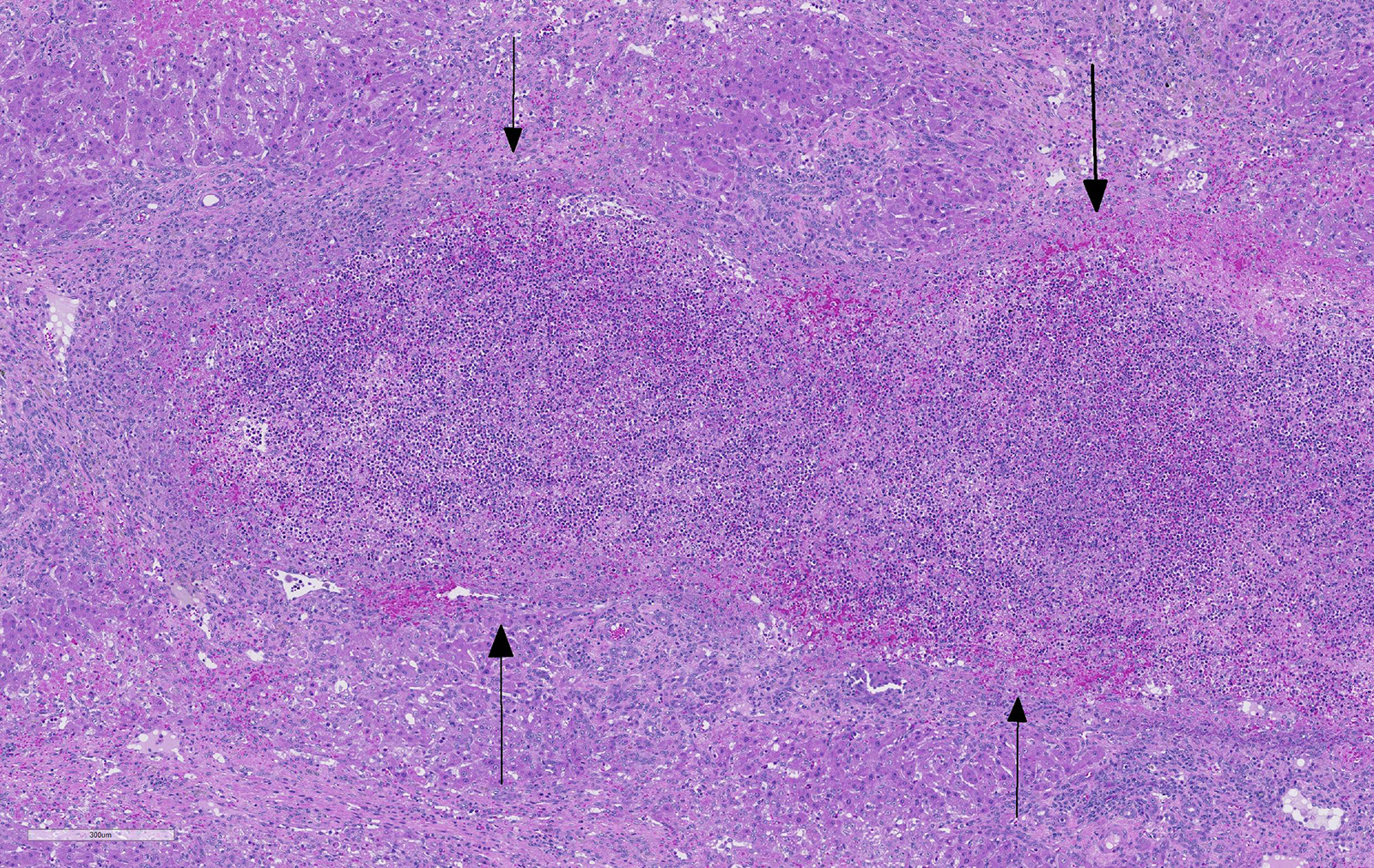
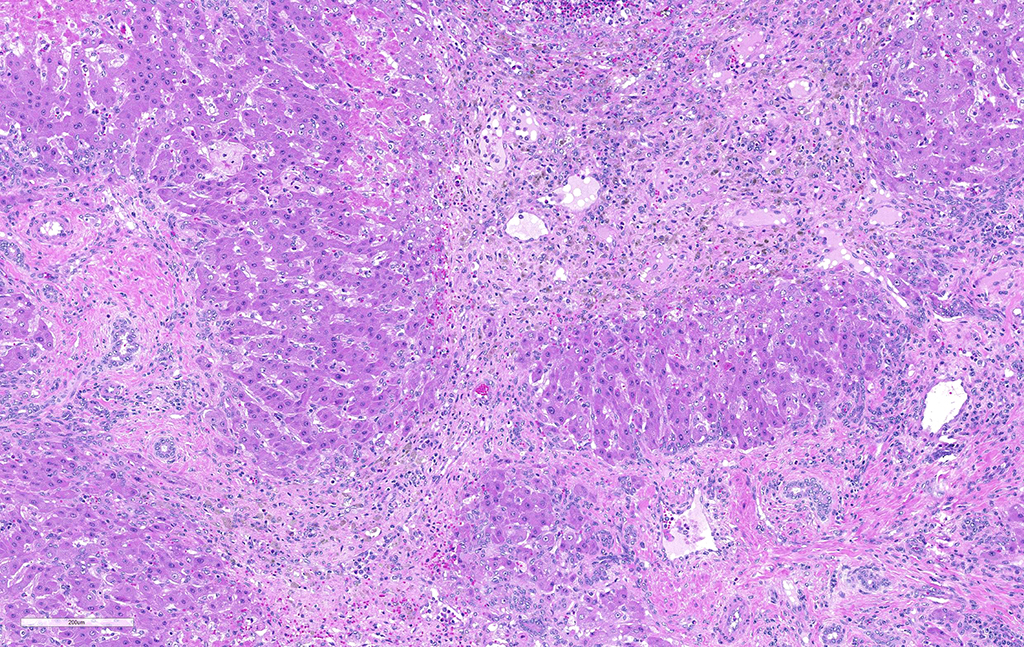
Liver: areas of coagulative necrosis and extensive hemorrhage in streaks or foci and disruption of the parenchyma with neutrophil and eosinophil infiltration. There was also fibrosis and bili duct hyperplasia in some areas. Immature forms of F. hepatica were observed in the parenchyma surrounded by degenerated hepatocytes, neutrophil, eosinophil and hemorrhagia.
Additional findings (organs not submitted): peritonitis with fibrinohemorrhagic deposits on the serous surfaces of abdominal cavity. Multifocal fibrosis, hemorrhagia and neutrophils infiltration were observed in renal cortex and some hyaline casts was present in renal tubules.
Contributor’s Morphologic Diagnoses:
Hepatitis with extensive hemorrhages associated with infiltration of neutrophils and eosinophils and the presence of immature forms of F. hepatica. Fibrosis and bile duct hyperplasia
Contributor’s Comment: Fasciolosis is a common parasitic disease of domestic ruminants throughout the world. The disease is caused most commonly by the trematode Fasciola hepatica that has a cosmopolitan distribution.10 Liver fluke infections affect farm animals especially in terms of poor productivity, reduced milk yield and livers condemned at slaughter11. F hepatica remains an economically significant parasite of livestock and is emerging as an important zoonotic infection of humans.6 In southern Brazil, the rate of liver condemnation at slaughter due to infection by F. hepatica is 10.34% for cattle and 20% for buffaloes5, 38% for cattle and 7% for sheep.2
Infection occurs by ingestion; excystment occurs in the duodenum. The young flukes penetrate the intestinal wall and cross the peritoneal cavity, attaching here and there to suck blood and penetrate the liver through its capsule.10 The acute phase follows and coincides with active liver migration of immature parasites.6 Parasites digest hepatic tissue and cause extensive parenchymal destruction with intensive hemorrhagic lesions and immunological reactions. On the other hand, the injury of the liver can be also induced chemically by factors produced or induced by the fluke. Immature flukes wander in the liver for a month or more before settling down in the bile ducts to mature, which they do in 2-3 months.10 The chronic phase begins 30 to 40 days after exposure as the parasites enter the bile ducts.6
Usually there is no obvious reaction to the passage of young flukes through the intestinal wall and across the peritoneal cavity and parasites may be found in ascitic fluid. Acute and exudative or chronic and proliferative peritonitis occurs in heavy and repeat infections.10
Wet environments with temperature between 10-25°C are necessary for proliferation of the parasite as well the intermediate host (snail).9 Precipitation may favor the accumulation of water that is a prerequisite for the disease cycle. Generally, this buildup occurs in flat or less mountainous terrain, as in Santa Vitória city (latitude S32º58'50.4"), extreme southern of Brazil, where this case occurred.
This case occurred in a recognized endemic area.5 Acute outbreaks are not common in cattle of this region where outbreaks with high mortality are only common in sheep.4 It seems possible that cattle from this area shows a relative resistance to the agent. A long time ago, resistance to F. hepatica infection in cattle has been reported.9
In the present case, the disease probably occurred as a result of consecutive infections since cows remained for a long period in a snail infected wetland on the farm. However, the cattle had been treated in May and September with drugs of nitroxynil group, it is possible that these drugs cannot be effective, since does not act in the young forms of the parasite. Moreover, F. hepatica resistance has been reported to these drugs.7,8 In mice secondary infections, the inflammatory response was faster and shorter. Resolution also was more rapid and often complete in 30 days.6
Contributing Institution:
Animal Pathology Department / Veterinary Diagnostic Laboratory, Veterinary Faculty; Federal University of Pelotas. 96010-900 Pelotas, RS, Brazil.
http://www.ufpel.edu.br/fvet/oncovet/
http://www.ufpel.edu.br/fvet/lrd/
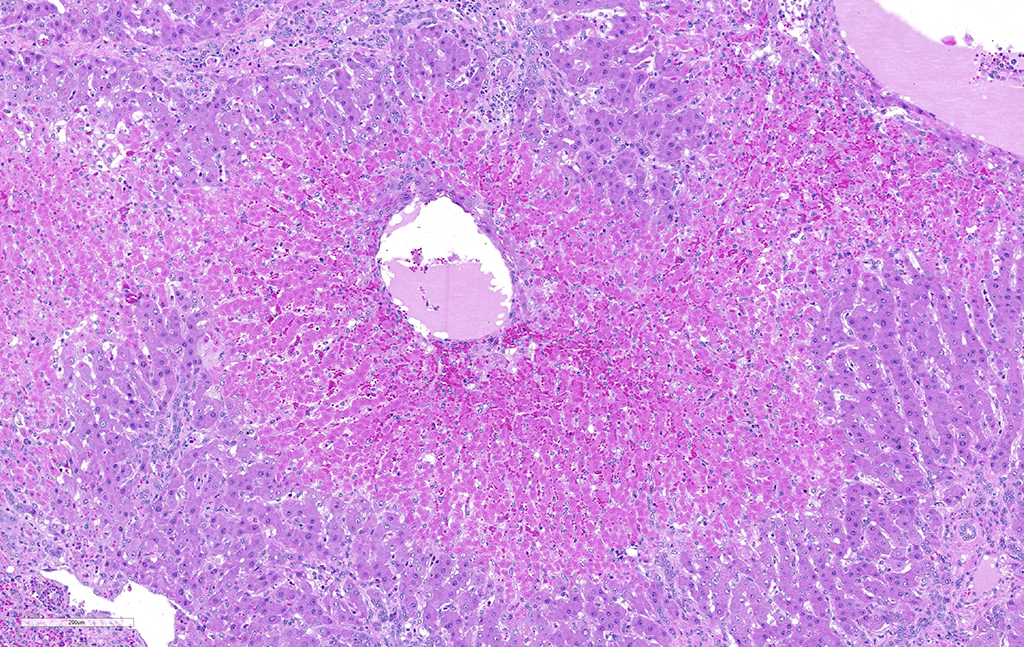
JPC Diagnosis: Liver: Fibrosis, portal, bridging and random, diffuse, moderate to severe, with arteriolar thrombosis, larval trematodes, and migration tracts
- Liver, centrilobular hepatocytes: Necrosis, multifocal.
JPC Comment: The contributor does an excellent job of describing the pathogenesis and clinical issues surrounding F. hepatica in cattle. While most commonly associated with infection in ruminants, F. hepatica (and a second related species, F. gigantea) can result in fascoliasis in a much wider range of species, including pigs, dogs, cats, capybara, and humans. (WSC 2010-2011, Conference 10 Case 1, details a case of F. hepatica in a pony from Ireland.)
F. hepatica, termed the temperate fluke, is found on every inhabited continent, while F. gigantean is found in tropical areas of Asia and Africa.3 The life cycle (as described above) is similar for both flukes. Interestingly, in areas of Japan, Vienam, and Korea, a number of intermediate hybrids between the two species have been identified by morphometric and molecular analysis. 3
Two possible sequelae to hepatic trematodiasis are black disease (C. novyi) in sheep and bacillary hemoglobinuria (C. hemolyticum) in sheep and cattle. The migration of immature flukes through the hepatic parenchyma may result in the generation of necessary ischemic conditions for the proliferation of C. novyi spores, already within the liver, to proliferate. Once active, C. novyi produces a necrotizing beta toxin and a hemolytic phospholipase C, resulting in large areas of coagulative necrosis in the liver and death, often with no premonitory signs. The pathogenesis of bacillary hemoglobinuria, caused by Clostridium haemolyticum, is similar to black disease with respect to the fluke involved, germination of spores, and toxin production. The toxins of C. haemolyticum also produces intravascular hemolysis with associated anemia and hemoglobinuria.
Fascioliasis is a tropical disease in humans whose prevalence is likely underestimated given the lack of surveys in endemic areas. Humans may be infected as a result of ingesting metacercariae-infected water or aquatic salad vegetation, such as watercress.3 Two main phases exist in the human disease. The acute phase of the disease coincides with the migration of immature flukes through liver liver, One to three months following ingestion of the metacercariae, the course of disease is marked by fever, right quadrant pain, hepatomegaly, eosinophilia and hypergammaglobulinemia. The second, or biliary stage, corresponds to active cholangitis with or without cholestasis, andthe presence of flukes within biliary radicles.1 Diagnosis may be accomplished by stool examination for ova and parasites, but is unreliable during acute stages. The most widely used diagnostic technique is an ELISA test for antibodies against secretory/excretory products from the flukes themselves. Ultrasound or CT results may demonstrate the hyperplastic changes in bile ducts and periductal fibrosis, as well as capsular fibrosis and subcapsular hemorrhage, changes quite similar to that seen in affected animals.1 While generally easily treated with anti-fluke drugs such as triclabendazole and bithionol, developing resistance to these drugs poses a significant problem for both animals and humans and poses a “One Health” problem for veterinarians and physicians alike.
There is significant slide variation in this case, with not all sections having the arteriolar thrombosis and areas of coagulative centrilobular necrosis. The etiology of the centrilobular necrosis was largely attributed to vascular insufficiency resulting from arteriolar thrombosis, however the possibility of shock as a cause could not be completed excluded.
References:
- Aksoy DY, Kermolglu U, Oto A, Erguven S, Arslan S, Unal S, Batman F and Bayraktar Y. Infection with Fasciola hepatica. Clin Microbiol Infect 2005: 11:859-861.
- Cunha FOV, Marques SMT, Mattos MJT. Prevalence of slaughter and liver condemnation due to Fasciola hepatica among sheep in the state of Rio Grande do Sul, Brazil 2000 and 2005. Parasitol Latinoam. 2007; 62: 188 - 191
- Cwiklinski K, O’Neill SM, Donnelly S, Dalton JP. A prospective view of animal and human fasciolosis. Parasite Immunol 2016; 38:558-568.
- Fairweather, I. 2011. Reducing the future threat from (liver) fluke: realistic prospect or quixotic fantasy? Vet. Parasitol. 180: 133-143
- Marques, S.M., Scroferneker, M.L. 2003. Fasciola hepatica infection in cattle and buffaloes in the state of Rio Grande do Sul, Brazil. Parasitol Lationam. 58: 169-172
- Masake RA, Wescott RB, Spencer GR, Lang BZ. The pathogenesis of primary and secondary infection with Fasciola hepatica in mice. Vet Pathol. 1978; 15: 763-769
- Moll L, Gaasenbeek CPH, Vellema P, Borgsteede FHM. Resistance of Fasciola hepatica against triclabendazole in cattle and sheep in The Netherlands. Vet Parasitol. 2000; 91: 153–158
- Olaechea F, Lovera V, Larroza M, Raffo F, Cabrera R. Resistance of Fasciola hepatica against triclabendazole in cattle in Patagonia (Argentina). Vet Parasitol. 2011; 178: 364–366
- Smithers SR, Terry RJ. Resistance of the host to Fasciola hepatica. Proc. R. Soc. Med. 1967; 60:168-169
- Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 5th ed., vol. 2. Philadelphia, PA: Saunders Elsevier; 2007:359-362
- van Dijk, J, Sargison, ND, Kenyon, F, Skuce, PJ. Climate change and infectious disease: helminthological challenges to farmed ruminants in temperate regions. Animal. 2010. 4(3): 377–392