Signalment:
Gross Description:
Histopathologic Description:
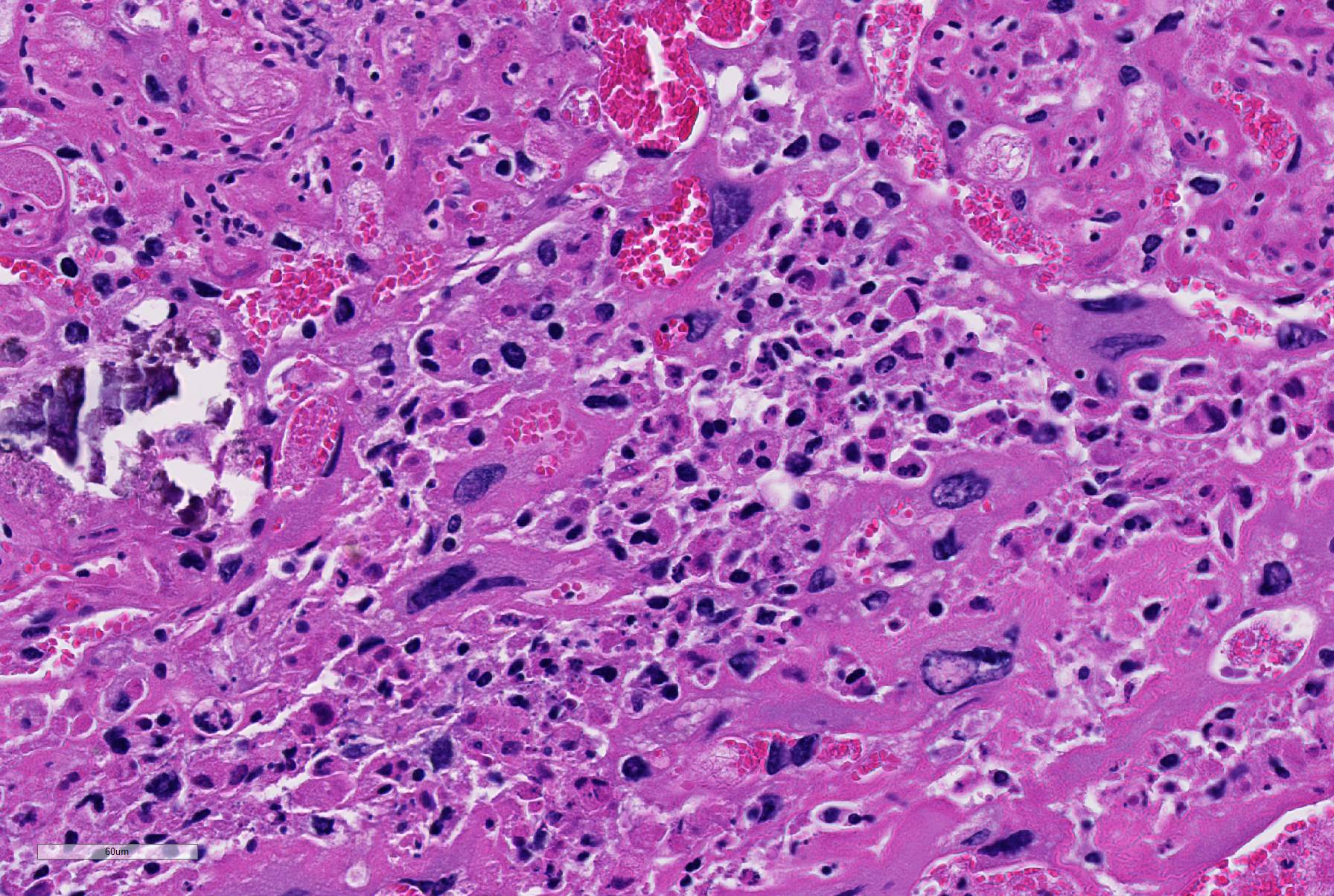
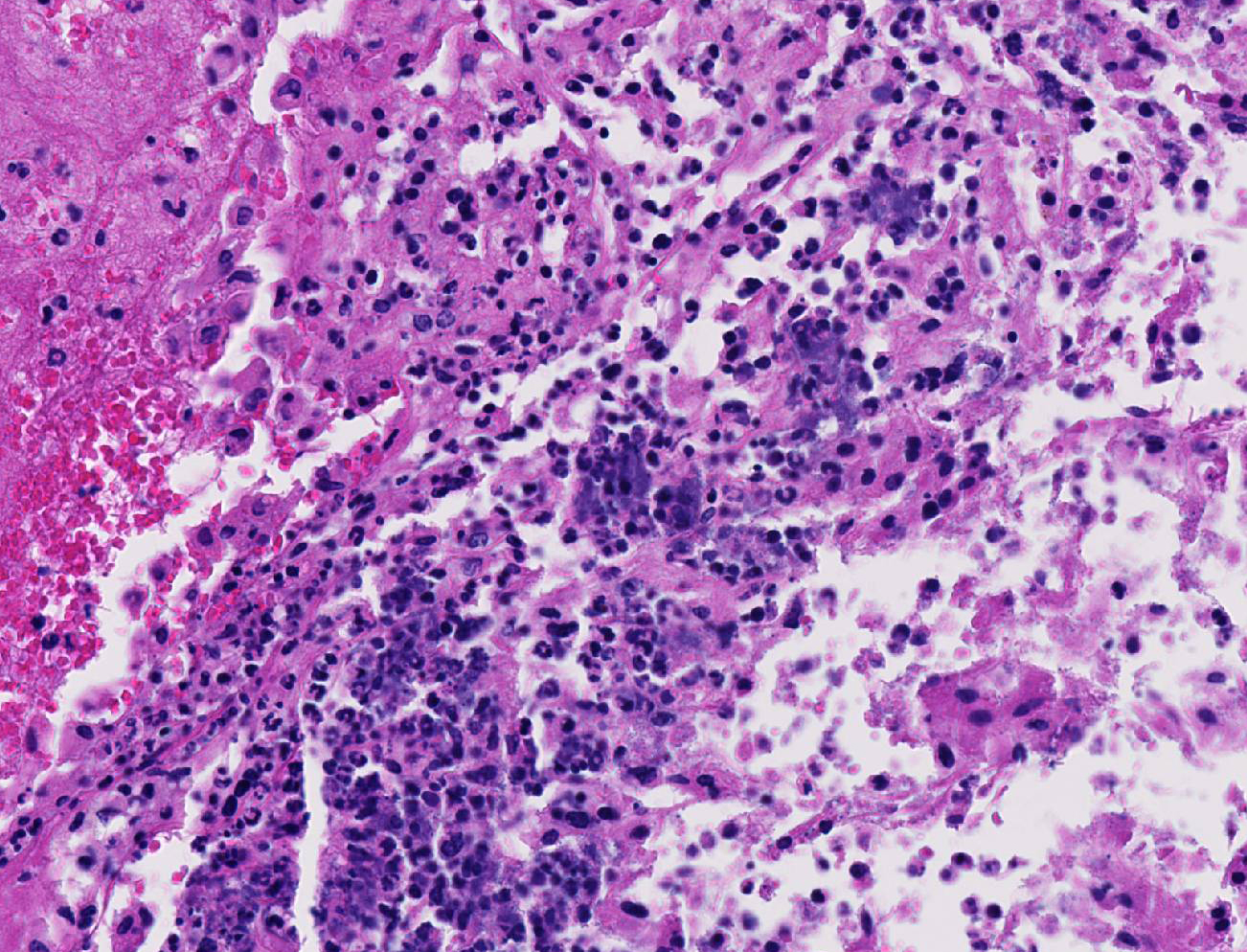
There was moderate to severe multifocal necrosuppurative endometritis with bacterial colonies present associated with the uterine inflammation. One of the two fetuses examined had moderate autolysis consistent with death in utero, with no evidence of inflammation or bacteria. No other significant lesions were noted.
Morphologic Diagnosis:
2. Placental labyrinth capillaries, mineralization, diffuse, moderate.
3. Placental membranes, amniochorionitis, necrosuppurative, acute, moderate to severe
Lab Results:
Condition:
Contributor Comment:
Pasteurella pneumotropica is generally considered an opportunistic agent and some degree of immunodeficiency often plays a role in disease pathogenesis.8 It has been isolated from the skin, conjunctiva, nasopharynx, trachea, lung, intestinal tract, vagina, uterus, urinary bladder, prepuce, preputial glands, bulbourethral glands and seminal vesicles in asymptomatic and clinically affected mice.6,13 Infections have been reported in mice, rats, guinea pigs, hamsters, cotton rats, rabbits, dogs, cats and humans.1 The agent can infect the vaginal tract and ascend to the uterus causing infertility associated with metritis, abortion and stillbirths.1
The primary route of infection is by direct contact with oropharyngeal or reproductive tract secretions.12 Mice and rats with infected uteri are typically found in colonies known to have a positive incidence of Pasteurella pneumotropica in the upper respiratory tract.3 Bacterial toxins PnxlA,llA and lllA have been identified on infected cell surfaces and are associated with adhesion to extracellular matrix and virulence. These toxins may also act as leukotoxins and disrupt actin cytoskeleton.11
Treatment in drinking water with enrofloxacin (Baytril) a broad spectrum, bactericidal, fluoroquinolone antibiotic has been used effectively to eliminate Pasteurella pneumotropica from infected mice.12 The finding of diffuse mineralization of placental labyrinth capillaries was interesting. This may be a manifestation of dystrophic calcification, and associated ineffective placental circulation may be a direct cause of fetal death in utero in this case.
JPC Diagnosis:
Conference Comment:
Subjacent to the labyrinth zone is the basal zone which is composed of spongio-trophoblasts, trophoblastic giant cells, and glycogen cells. Spongiotrophoblasts are located at the maternal and fetal interface between the trophoblastic giant cell and inner labyrinth layer.4 The trophoblastic giant cells are large pleomorphic cells that show extensive DNA replication and can have the equivalent of up to 1000 copies of the genome within a single cell.4,6 These cells are vital for implantation and secretion of a wide array of hormones. Care should be taken to not interpret these cells as anaplastic tumor giant cells.6 Glycogen cells have abundant intracytoplasmic glycogen and usually disappear prior to parturition. The origin and function of the glycogen cells is unknown. The decidua is composed of the maternal endometrial lining cells that are responsible for the exchange of nutrients, gas, and waste products between the fetus and the dam.2,4,6 Finally, the metrial gland is present in the mesometrial triangle of the gravid mouse uterus and contains granulated endometrial gland cells (GMG), endometrial stromal cells, blood vessels, trophoblasts, and fibroblasts. Granulated mesometrial gland cells are bone marrow derived perforin-positive, natural killer cells that proliferate within the metrial gland upon pregnancy in mice. In health, GMG are mitotically active and often binucleated. The exact function of these cells has not yet been fully elucidated.4,10
Conference participants noted some moderate slide variation in this case, as some sections contain only the placental disk with no sections of the placental membrane or attached uterus. Additionally, the dystrophic mineralization of the capillaries within the placental labyrith, mentioned by the contributor, is not as prominent on every examined slide. Conference participants readily identified areas of lytic necrosis with moderate numbers of infiltrating neutrophils centered on small colonies of coccobacilli. Pasteurella pneumotropica is a common opportunistic pathogen in mice and infection often produces subclinical disease in immune competent mice.9 The increased use of immunosuppressed strains of mice, however, has contributed to an increase in the incidence of clinical disease. Typically, it is implicated as a cause of severe pneumonia (hence its name), but has been rarely reported as a cause of a wide variety of necrotizing and suppurative lesions, including those leading to infertility, reproductive failure and abortion, as in this case.9 Most immunocompetent rats and mice with clinical respiratory disease are co-infected with Sendai virus, Mycoplasma pulmonis, cilia-associated respiratory (CAR) bacillus, Streptococcus pneumoniae, Corynebacterium kutscheri, Bordetella bronchiseptica, or Klebsiella pneumoniae as part of the chronic respiratory disease syndrome.9
References:
1. Ackerman JI, Fox JG. Isolation of Pasteurella ureae from reproductive tracts of congenic mice. J Clin Microbiol. 1981; 13(6):1049-53.
2. Bacha WJ, Bacha LM. Color Atlas of Veterinary Histology. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2012:243-260.
3. Blackmore DK, Casillo S. Experimental investigation of uterine infections of mice due to Pasteurella pneumotropica. J Comp Pathol. 1972; 82(4):471-5.
4. Furukawa S, Kuroda Y, et al. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014; 27(1):11-18.
5. Hooper A, Sebesteny A. Variation in Pasteurella pneumotropica. J Med Microbiol. 1974; 7(1):137-40.
6. Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol. 2010; 54(3):341-354.
7. Kawamoto E, Sasaki H, Okiyama E, Kanai T, Ueshiba H, Ohnishi N, Sawada T, Hayashimoto N, Takakura A, Itoh T. Pathogenicity of Pasteurella pneumotropica in immunodeficient NOD/ShiJic-scid/Jcl and immunocompetent Crlj: CD1 (ICR) mice. Exp Anim. 2011;60(5):463-70.
8. Matsumiya LC, Lavoie C. An outbreak of Pasteurella pneumotropica in genetically modified mice: Treatment and elimination. Contemp Top Lab Anim Sci. 2003; 42(2):26-8.
9. Percy DH, Barthold SW. Mouse. In: Pathology of Laboratory Rodents and Rabbits, 4th ed. Ames, IA: Blackwell Publishing; 2016:66-67.
10. Picut CA, Swanson CL, et al. The metrial gland in the rat and its similarities to granular cell tumors. Toxicol Pathol. 2009; 37(4):474-480.
11. Sasaki H, Ishikawa H, Sato T, Sekiguchi S, Amao H, Kawamoto E, Matsumoto T, Shirama K. Molecular and virulence characteristics of an outer membrane-associated RTX exoprotein in Pasteurella pneumotropica. BMC Microbiol. 201; 11:55.
12. Towne JW, Wagner AM, Griffin KJ, Buntzman AS, Frelinger JA, Besselsen DG. Elimination of Pasteurella pneumotropica from a mouse barrier facility by using a modified enrofloxacin treatment regimen. J Am Assoc Lab Anim Sci. 2014; 53(5):517-22.
13. Ward GE, Moffatt R, Olfert E.J. Abortion in mice associated with Pasteurella pneumotropica. Clin Microbiol. 1978; 8(2):177-80.