CONFERENCE 24, CASE 3
History:
Six out of fifty 2–3-week-old, Angus calves were found dead with other calves presenting with lethargy, tachypnea, hypersalivation, circling, tremors, and seizures, with rapid deterioration preceding to death. Calves were otherwise in good body condition. No abnormalities of calves were observed at birth. These calves originated from artificially inseminated heifers.
Laboratory Results:
Bovine herpesvirus real time PCR positive on fresh brain
Bovine herpesvirus-5 real time PCR positive on fresh brain
Listeria spp. selective culture on fresh brain negative
Chlamydia pecorum real time PCR on brain dry swab negative
Aqueous humor urea, glucose, β-hydroxybutyrate, calcium, magnesium, nitrate, nitrite analytes within normal limits. Aqueous humor potassium is elevated (likely due to delayed post-mortem sampling or vitreous contamination).
Contributor’s Microscopic Description:
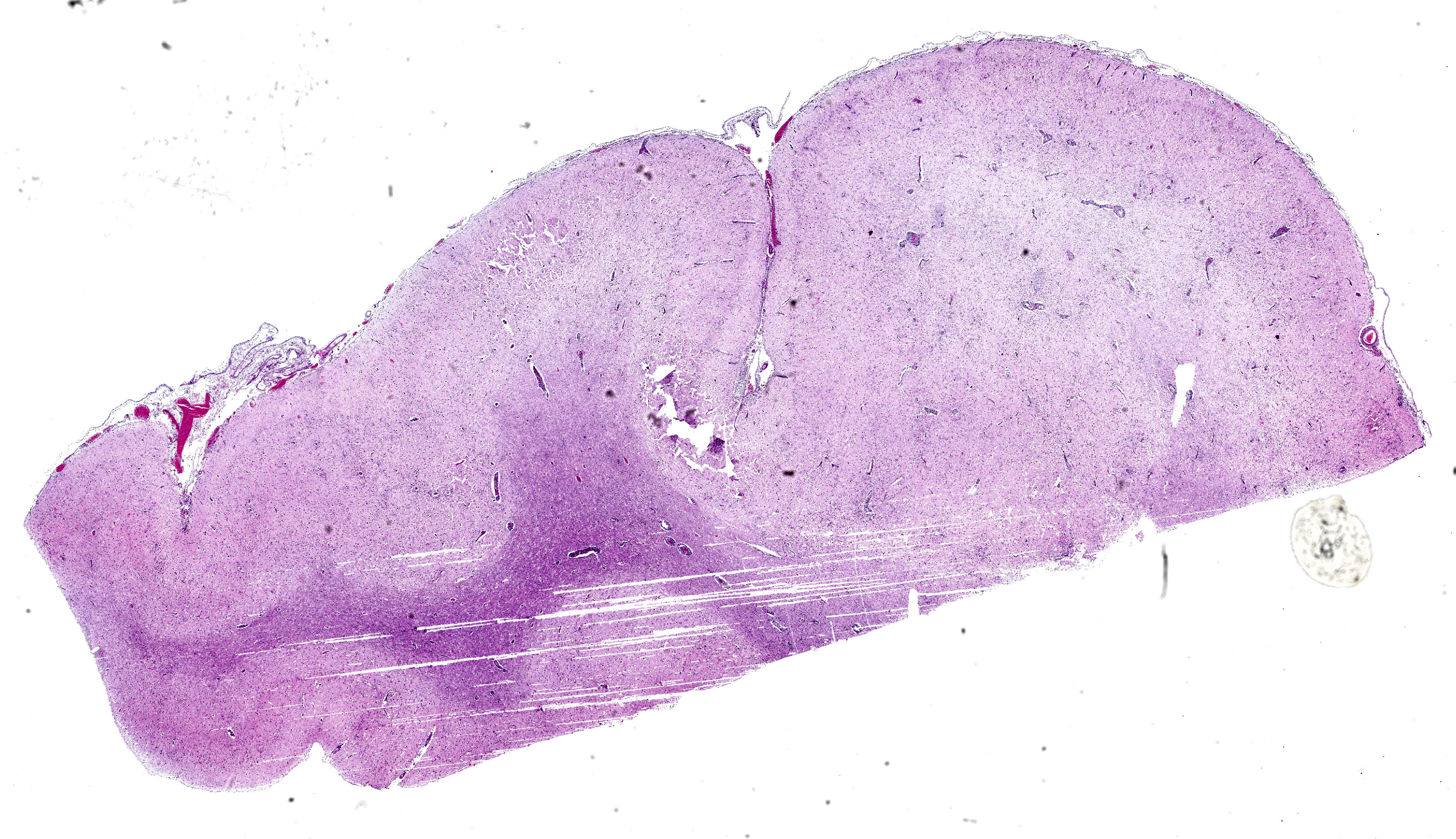
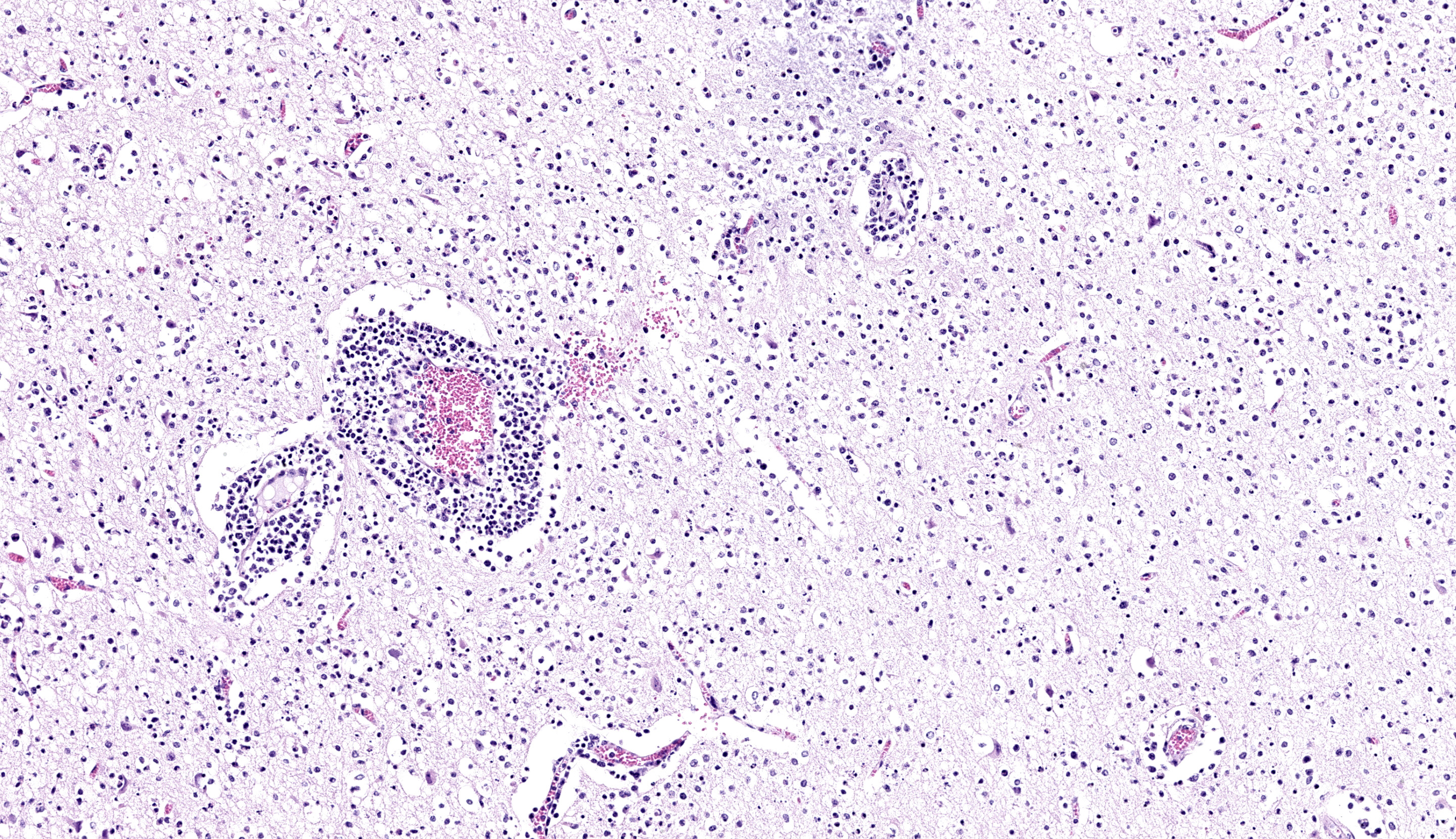
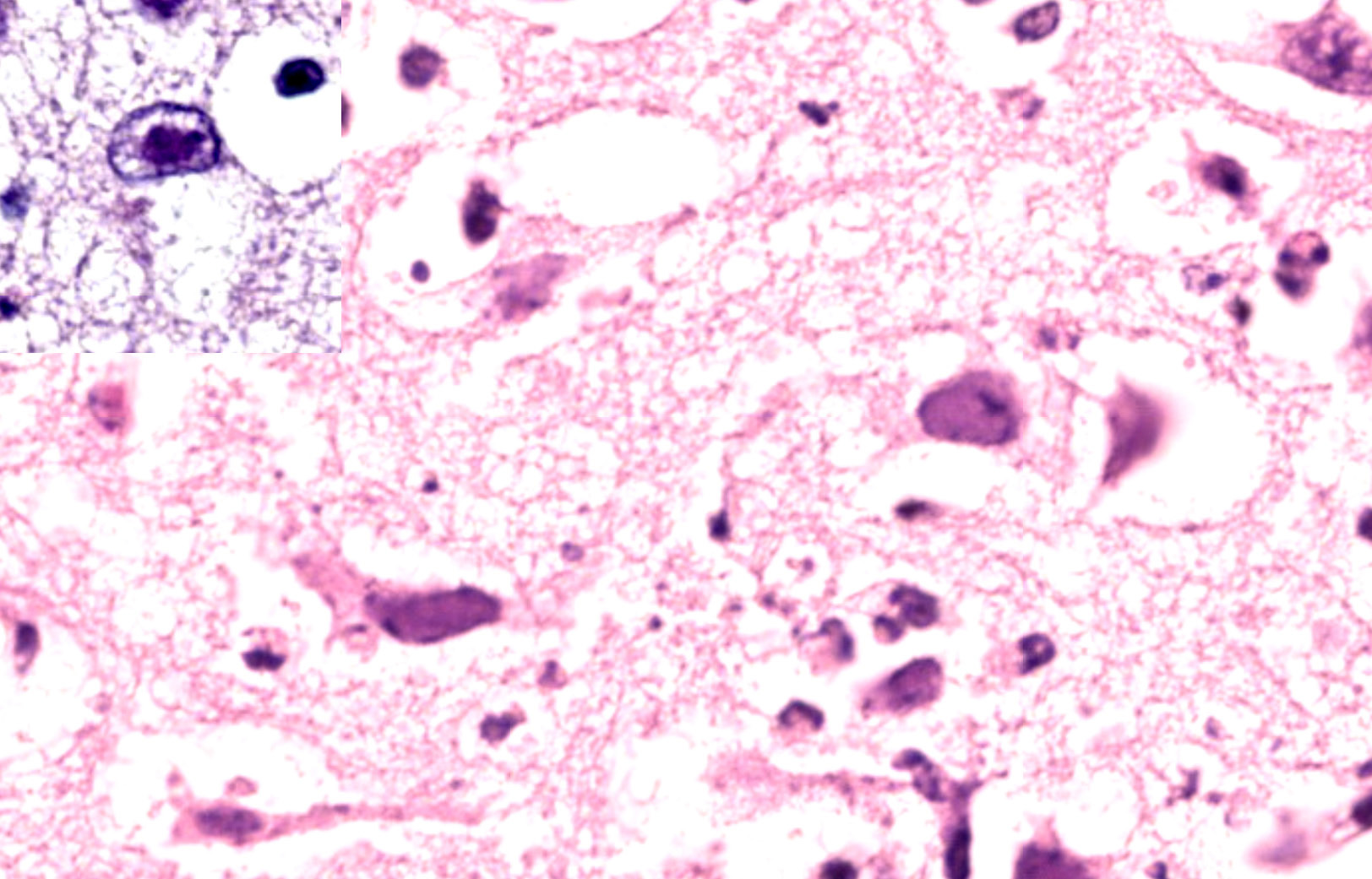
Brain; rostral cerebrum: Multifocally, the meninges contain small numbers of lymphocytes, occasional macrophages and extravasated erythrocytes (hemorrhage), and rare neutrophils. Frequently, blood vessels in the white and to a lesser extent the gray matter are cuffed by up to 4-5 layers of lymphocytes, macrophages, and rare plasma cells. Occasional scattered neurons are shrunken, angular, hypereosinophilic, and have pyknotic or karyorrhectic nuclei (neuronal necrosis), rarely surrounded by up to 5 glial cells (satellitosis) and karyorrhectic nuclear debris. Multifocal patches in the gray matter have moderate numbers of neurons and rare astrocytes contain large, basophilic to amphophilic, intranuclear viral inclusions which peripheralise the chromatin (. Multifocally in the gray and white matter, there are abundant glial cells (gliosis), with astrocytes with vesiculated nuclei, which are often paired or clustered, frequently with karyorrhectic nuclei (glial cell necrosis) with large amounts of karyorrhectic nuclear debris scattered throughout the neuropil. Extensively within the gray matter and extending into the white matter, blood vessels, neurons, and glial cells are surrounded by clear spaces (edema) with frequent small, irregular clear spaces expanding the neuropil (spongiosis).
Contributor’s Morphologic Diagnosis:
Brain, rostral cerebrum: Meningoencephalitis, lymphohistiocytic, subacute, multifocal, severe with multifocal neuronal and glial cell
Contributor’s Comment: Bovine herpesvirus-5 (BoHV-5) is an alphaherpesvirus known to cause meningoencephalitis in young cattle. BoHV-5 is closely related to BoHV-1 (the causative agent of bovine infectious rhinotracheitis) and was originally considered to be a “neurogenic variant of BoHV-1” or “BoHV-1.3”.4 After further comparative studies with different strains of BoHV-1, it was determined that this virus had distinct and differing genomic and antigenic properties and was reclassified as BoHV-5 by the International Committee on Taxonomy of Viruses in 1992.4,10 BoHV-5 encephalitis outbreaks have been reported mostly in South American countries such as Brazil and Argentina, but also sporadically in other countries including Australia, and within North America and Europe.4,9 BoHV-5 is part of a group of herpesviruses known to cause specific disease syndromes in bovines summarized in table 1 below.
BoHV-5 associated encephalitis is a typically a sporadic disease, but occasional outbreaks can occur in groups of calves and yearlings.7 Morbidity can reach up to 50% and few symptomatic individuals survive.7 The incubation period is approximately 1-2 weeks followed by presentation of clinical signs including depression, anorexia, weakness, proceeding to neurological signs such as circling, head pressing, incoordination, blindness, muscular tremors, convulsions, paddling, and death.4,7 Nasal and ocular discharges have also been reported in some outbreaks of BoHV-5 associated disease, mimicking the classical clinical presentation of BoHV-1.7
Both BoHV-1 and -5 have many commonalities in their pathogenesis including the ability to infect epithelial cells at the portal of entry, establish latency in the trigeminal ganglia, and can be reactivated by natural or experimental stressors.4,7 BoHV-5 associated disease differs from that of BoHV-1 as they have different neuroinvasion and neurovirulence capabilities with BoHV-1 rarely causing encephalitis.7 The exact pathogenesis of BoHV-5 is unknown. Transmission occurs via direct contact, aerosolization, or indirect spread through contaminated feed and water, or semen.4,7 Following inoculation, BoHV-5 infects and replicates within epithelial cells at the portal of entry (e.g., nasal or vaginal mucosa).4 Several possibilities for BoHV-5 neural invasion are being investigated including local spread from infected nasal epithelial cells with intra-axonal transport via the trigeminal or olfactory pathways, or by hematogenous viremic spread.4,6 Once BoHV-5 has gained entry to the central nervous system, it can cause acute meningoencephalitis or become latent within the sensory ganglia, with possible reactivation and clinical recrudescence induced at times of stress.11
The characteristic histopathological changes associated with BoHV-5 are non-suppurative meningoencephalitis with mononuclear perivascular cuffing, gliosis, satellitosis, neuronophagia, trigeminal ganglioneuritis, and neuronal necrosis and degeneration.7 Malacia is reported variably across literature, can take a laminar cortical necrosis pattern of polioencephalomalacia (PEM), and is proposed to occur dependent on strain neurovirulence or individual susceptibility.1,3 Intranuclear alphaherpesviral inclusion bodies are occasionally present in degenerate neurons and astrocytes.7 Histopathological changes are most severe within the frontal cortex, with milder changes described variably within the diencephalon, parietal and occipital cortices, cerebellum, basal nuclei, and brain stem.2 In our case, milder changes were observed within the dorsal cerebrum, thalamus, and brainstem.
Possible histological differential diagnoses of BoHV-5 meningoencephalitis in calves include BoHV-1 infection, rabies, or malignant catarrhal fever, listeriosis, sporadic bovine encephalomyelitis (caused by Chlamydia pecorum), or less likely PEM causes such as lead or sulfur toxicity, thiamine deficiency, or salt poisoning.5
Table 1: Outline of bovine diseases associated with bovine herpes viral infections.
|
Bovine herpesvirus type |
Sub-family |
Disease associations |
|
BoHV-16-8 |
Alpha |
|
|
BoHV-26,7 |
Alpha |
|
|
BoHV-4 (“Bovine cytomegalovirus”)8 |
Gamma |
|
|
BoHV-56,7 |
Alpha |
|
Contributing Institution:
NSW Animal and Plant Health Laboratories
Elizabeth Macarthur Agricultural Institute Woodbridge Rd, Menangle
NSW, Australia, 2568
https://www.dpi.nsw.gov.au/about-us/services/laboratory-services/veterinary
JPC Morphologic Diagnosis: Cerebrum: Meningoencephalitis, lymphohistiocytic, subacute, diffuse, moderate, with neuronal necrosis and glial and neuronal intranuclear viral inclusions.
JPC Comment: The third case of this conference is yet another alphaherpesvirus and does not disappoint. Recent (and quite timely) publication12 reviewing bovine herpesviral meningoencephalitis was a welcome addition to our discussion. Gross images from that publication nicely highlight the predominant frontal lobe distribution of BoHV-5, to include the sometimes dramatic malacia that the contributor notes above. We considered many of the same differential diagnoses that the contributor helpfully notes, but would also add rabies (though 5 animals is a bit of a stretch), bovine astrovirus, West Nile virus, and listeriosis as other broader differentials for bovine meningoencephalitis. Dr. Pesavento emphasized low power evaluation of any brain section for symmetry, position of the midline, distribution of features (to include portion of the brain examined), and loss of parenchyma to help sort (or rule out) these possibilities. Intranuclear viral inclusions were fairly generous in this case which aided in recognition of the etiological agent.
Mild neutrophilic inflammation was an unexpected finding in this calf’s brain given the primary viral etiology. We considered a secondary process such as sepsis, though there is little lymphoid necrosis present within perivascular lymphoid cuffs and neutrophils are well within the parenchyma itself. One potential explanation is the young age (14 days) of this animal and insufficient time course to develop and deploy other innate/adaptive immune cells in large numbers akin to some of the NOD/Rag1/ILrg2 fully immunodeficient mice we have considered in conference this year (see Conference 11, Case 3 for one such example). We anticipate that in a slightly older animal histopathologic features would reflect non-suppurative meningoencephalitis alone.
Conference discussion of this case concluded with review of vascular features. In comparison to Case 4, this brain lacked changes in major blood vessels, though there was capillary damage coincident with eosinophilic fluid which we interpreted as true edema to accompany the spongiosis noted (versus processing artifact alone). Dr. Pesavento reminded conference goers that Factor VIII IHC is a potentially useful IHC marker for vascular damage as it also labels platelets, with the resulting perivascular aggregate serving as a reliable label when other markers (e.g. CD31) might be difficult to interpret alone.
References:
- Belknap EB, Collins JK, Ayers VK, Schultheiss PC. Experimental-Infection of Neonatal Calves with Neurovirulent Bovine Herpesvirus Type-1.3. Veterinary Pathology. 1994;31: 358-365.
- Cagnini DQ, Cunha PHJ, Pantoja JCF, et al. Histopathological, immunohistochemical, and molecular study of BHV-5 infection in the central nervous system of experimentally infected calves. Pesqui Vet Brasil. 2015;35: 337-343.
- David N, Huebner SO, Riet-Correa F, Halfen D, Lemos RA. Reactivation of latent bovine herpesvirus type 5 in cattle with polioencephalomalacia induced by ammonium sulphate. Pesqui Vet Brasil. 2007;27: 435-441.
- Del Medico Zajac MP, Ladelfa MF, Kotsias F, et al. Biology of bovine herpesvirus 5. Vet J. 2010;184: 138-145.
- Dore V, Smith G. Cerebral Disorders of Calves. Vet Clin North Am Food Anim Pract. 2017;33: 27-41.
- Engels M, Ackermann M. Pathogenesis of ruminant herpesvirus infections. Vet Microbiol. 1996;53: 3-15.
- Jubb KVF, Kennedy PC, Palmer N. Pathology of domestic animals, 6th ed., vol. 1. St. Louis: Elsevier; 2016.
- Jubb KVF, Kennedy PC, Palmer N. Pathology of domestic animals, 6th ed., vol. 3. St. Louis: Elsevier; 2016.
- Kessell A, Finnie J, Windsor P. Neurological diseases of ruminant livestock in Australia. IV: viral infections. Aust Vet J. 2011;89: 331-337.
- Roizmann B, Desrosiers RC, Fleckenstein B, Lopez C, Minson AC, Studdert MJ. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol. 1992;123: 425-449.
- Vogel FS, Caron L, Flores EF, et al. Distribution of bovine herpesvirus type 5 DNA in the central nervous systems of latently, experimentally infected calves. J Clin Microbiol. 2003;41: 4512-4520.
- Santos BS, Lemos RAA, Rech RR, Barros CSL, Rissi DR. Bovine herpesviral meningoencephalitis: large case study and literature review. Journal of Veterinary Diagnostic Investigation. 2025;37(3):417-428.