Signalment:
Gross Description:
Histopathologic Description:
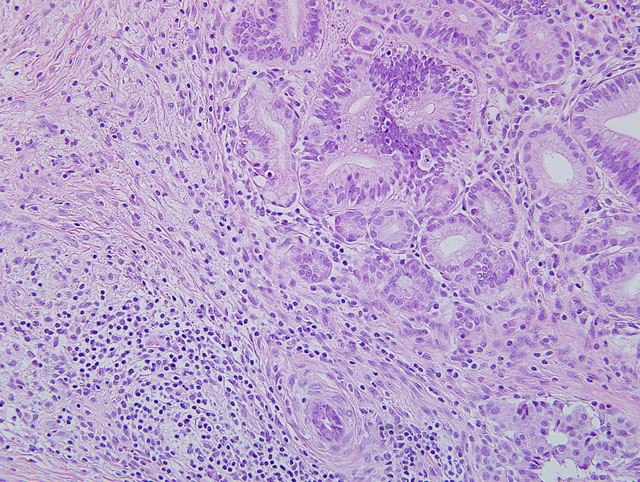
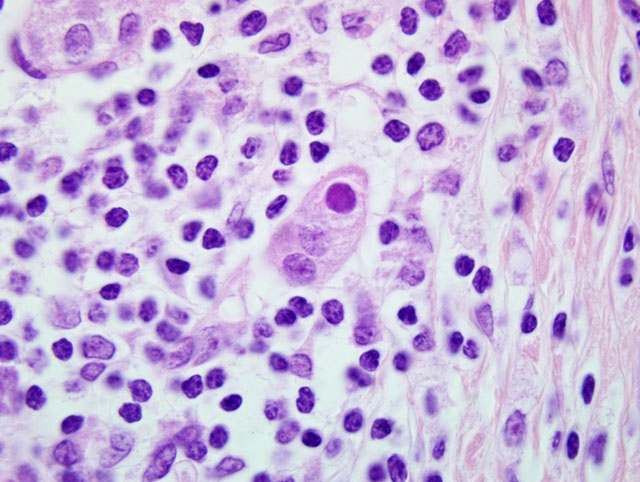
Pancreas: Replacing approximately 90% of exocrine pancreatic tissue, sparing only the main pancreatic duct, are multifocal to coalescing areas of necrosis composed of degenerate epithelial cells that are admixed by deposits of fibrin, cellular debris, and fibrosis that often encircle remnant islets of Langerhans. These areas of necrosis are characterized by large numbers of degenerate epithelial cells that have vacuolated cytoplasm and nuclear pyknosis [Figure 1]. Scattered acinar cells bordering the regions of necrosis contain prominent, 6-10 μm magenta, round, intranuclear inclusion bodies. Similar inclusion bodies are rarely noted within the ductular epithelium, almost exclusively in small degenerate epithelial cells that are partially exfoliated into the ductular lumen [Figure 2]. There is scattered hyperplasia of the remnant exocrine epithelial cells characterized by moderate anisocytosis and scattered mitotic figures. The fibrosis is loosely organized in many areas and is often interspersed with moderate to abundant numbers of lymphocytes and plasma cells with fewer macrophages and degenerate neutrophils. There are scattered hemosiderin-laden macrophages throughout the pancreas. In the peripancreatic fat and surrounding blood vessels within the adipose tissue there are small to moderate numbers of lymphocytes and plasma cells.
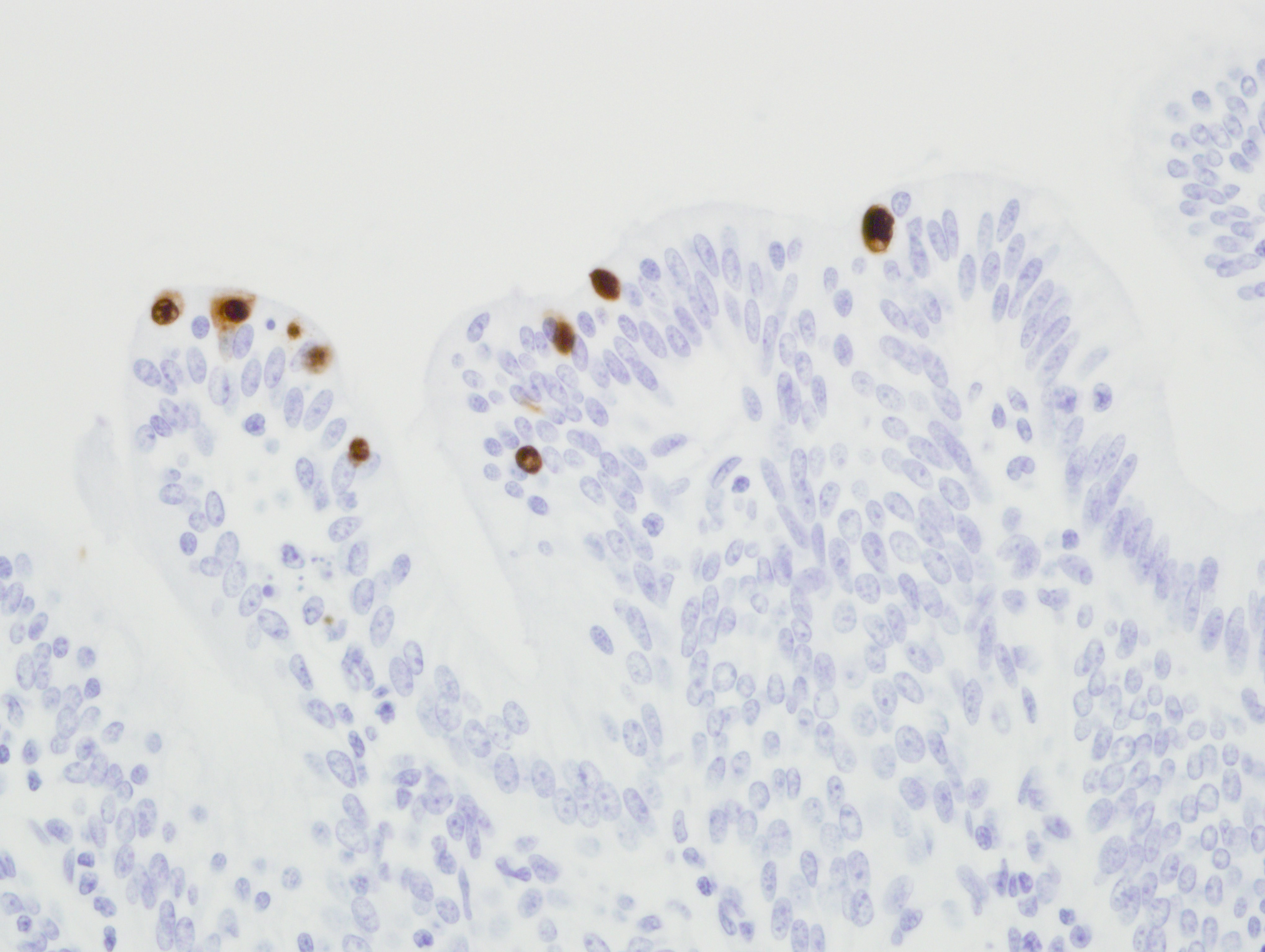
Immunohistochemistry for adenovirus: There are multifocal positive cells noted within the areas of necrosis and in the ductular epithelium. Immunoreactivity is strong and intranuclear. The isotype matched negative control revealed no immunoreactivity. [Fig 3]
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Adenoviruses are capable of establishing chronic infections following an initial exposure that may or may not be clinically relevant.(13) All of the adenoviruses have narrow host ranges. Many cause acute respiratory or gastrointestinal disease but also may cause persistent infection with periods of latency that become reactivated upon immunosuppresion.(6) In dogs and other canids, canine adenovirus-1 causes infectious canine hepatitis that produces acute necrosis and inflammation in the liver often accompanied by grossly visible edema of the gallbladder.4 Canine adenovirus-2 is one of the known causative agents of canine infectious tracheobronchitis, which is commonly referred as kennel cough.(9) Canine adenovirus type 2 can cause pneumonia in dogs which is clinically mild unless complicated with secondary bacterial infections.(9) Acute rhinitis that is manifested as part of general respiratory disease can be caused by both adenovirus type 1 and 2.(9) Adenoviruses are pneumotropic and enterotropic in ruminants.(6,9) Equine adenovirus type 1 (EAdV1) causes upper respiratory tract disease, follicular conjunctivitis, bronchopneumonia, and infection of the gastrointestinal tract. EAdV1 is peculiarly associated as a dominant pathogen in the uniformly fatal, inherited disease syndrome known as primary severe combined immunodeficiency disease (PSCID). The foals are born devoid of B and T lymphocytes and a consistent and dominant feature of PSCID is an inexorably progressive EAdV1 bronchopneumonia.(14) Mice are host to two distinct adenoviruses: mouse adenovirus type 1 (MAV-1), causing hemorrhagic foci of necrosis in multiple organs and wasting disease (especially in nude and SCID mice); and mouse adenovirus type 2 ( MAV-2), which is asymptomatic and does not exhibit clinical disease.(12)
In rhesus macaques, adenovirus infection is one of the most common opportunistic infections to accompany terminal cases of SIV infection. Adenoviral pancreatitis can also be seen in animals that are immunosuppressed for organ transplantation and in neonates. Adenovirus associated disease in rhesus macaques is most commonly found in the small and large intestine, the liver and gall bladder, and the pancreas. The pancreatic lesion is often fulminant with abundant necrosis making organ identification difficult. If the pancreatitis persists for long periods of time the marked epithelial hyperplasia and proliferation that occurs can be confused with a pre-neoplastic change. Chronic pancreatitis is characterized by destruction of exocrine tissue and is typically accompanied by fibrosis, parenchymal atrophy, and in late stages with destruction of endocrine parenchyma.(3,4) Chronic pancreatitis may present as repeated bouts of acute pancreatitis, the major distinction being an irreversible impairment of pancreatic function with the former. Chronic inflammation of the pancreas with mostly lymphoplasmacytic infiltrations is seen most commonly in the dog, but does occur in cat, horse and cattle.(4)
The most frequently reported pancreatic disease in nonhuman primates is diabetes mellitus secondary to the deposition of islet amyloid polypeptide (amylin) in the Islets of Langerhans. In addition, pancreatitis and islet destruction in nonhuman primates can be induced by certain drug treatments, such as streptozotocin, and interference with the pancreatic duct and blood supply.(10) Prior to the discovery of SIV as a major immunocompromising pathogen in rhesus macaques, spontaneous pancreatitis was reported rarely in nonhuman primates, and at least two cases were reported in rhesus monkeys with adenoviral inclusions.(1,2,5,10) The spontaneous nature of these early reports of adenoviral pancreatitis is dubious, as it is highly likely that an as yet unrecognized pathogen likely caused immunosuppresion predisposing to adenoviral disease in these animals. In cases of pancreatitis in nonhuman primates, especially rhesus macaques, it is imperative to determine if adenovirus played a role and if the animal was immunocompromised.
JPC Diagnosis:
Conference Comment:
Participants discussed the relationship between the location of viral inclusion bodies within the cell and the properties of the viral genome. In general, DNA viruses tend to replicate in the nucleus and produce intranuclear inclusion bodies, e.g. herpesviruses, adenoviruses, parvoviruses, etc. One notable exception to this generality is the Poxviridae family, which induce eosinophilic intracytoplasmic inclusion bodies. In the dog, few RNA viruses produce viral inclusion bodies visible by light microscopy, the most notable of which include: rabies virus, which results in the classic intracytoplasmic Negri body within infected neurons; and canine distemper virus, a morbillivirus that can result in both intranuclear and intracytoplasmic viral inclusion bodies.
The contributor provides an excellent overview of the viral properties of the Adenoviridae, comparative pathology of adenoviral infection, and pancreatic disease in nonhuman primates.
References:
2. Chandler FW, McClure HM. Adenoviral pancreatitis in rhesus monkeys: current knowledge. Vet Pathol. 1982;19:171-180.
3. Crawford JM. Liver and biliary tract. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2009:942-946.
4. Cullen JM. Liver, biliary system, and exocrine pancreas. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:440-443, 458-59.
5. Doepel FM, Anver MR, Hofing GL. Pancreatitis in two new world monkeys. Vet Pathol. 1980;17:505-508.
6. Geldberg HB. Alimentary system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:361.
7. Kalter SS. Enteric viruses of non-human primates. Vet Pathol. 1982;19:33-43.
8. Kovacs GM, Harrach B, Zakhartchouk AN, Davison AJ. Complete genome sequence of simian adenovirus 1: an Old World monkey adenovirus with two fiber genes. J Gen Virol. 2005;86:1681-1686.
9. Lopez A. Respiratory System. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:440-443, 522, 531.
10.McClure HM, Chandler FW. A survey of pancreatic lesions in non-human primates. Vet Pathol. 1982:19:193-209, 1982
11.Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Viral Taxonomy and Nomenclature. In: Veterinary Virology. 3rd ed. vol.1. San Diego, CA: Elsevier Academic Press; 1999:32
12.Percy DH, Barthold SW. Mouse. In: Pathology of Laboratory Rodents and Rabbits. 2nd ed. Ames, IA: Iowa State University Press; 2001:16-17.
13.Roy S, Vandenberghe LH, Kryazhimskiy S, et al. Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS pathogens. 2009;5:e1000503.
14.Studdert MJ. Equine Adenoviruses. In: Equine Respiratory Diseases. Ithaca, NY: International Veterinary Information Service (www.ivis.org); 2003.
15. Crawford JM: Liver and biliary tract. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Elsevier Saunders; 2009:942-946.