Signalment:
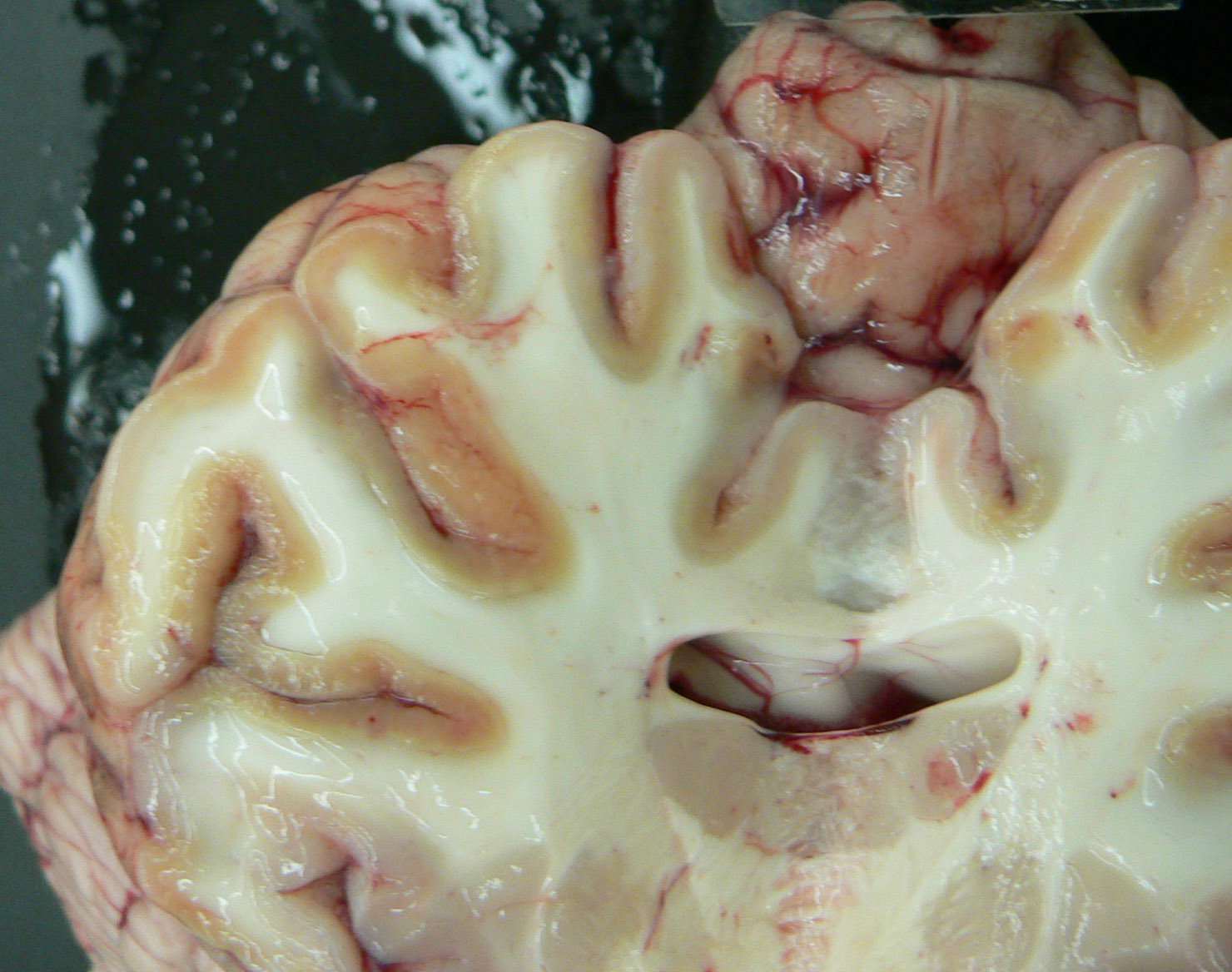
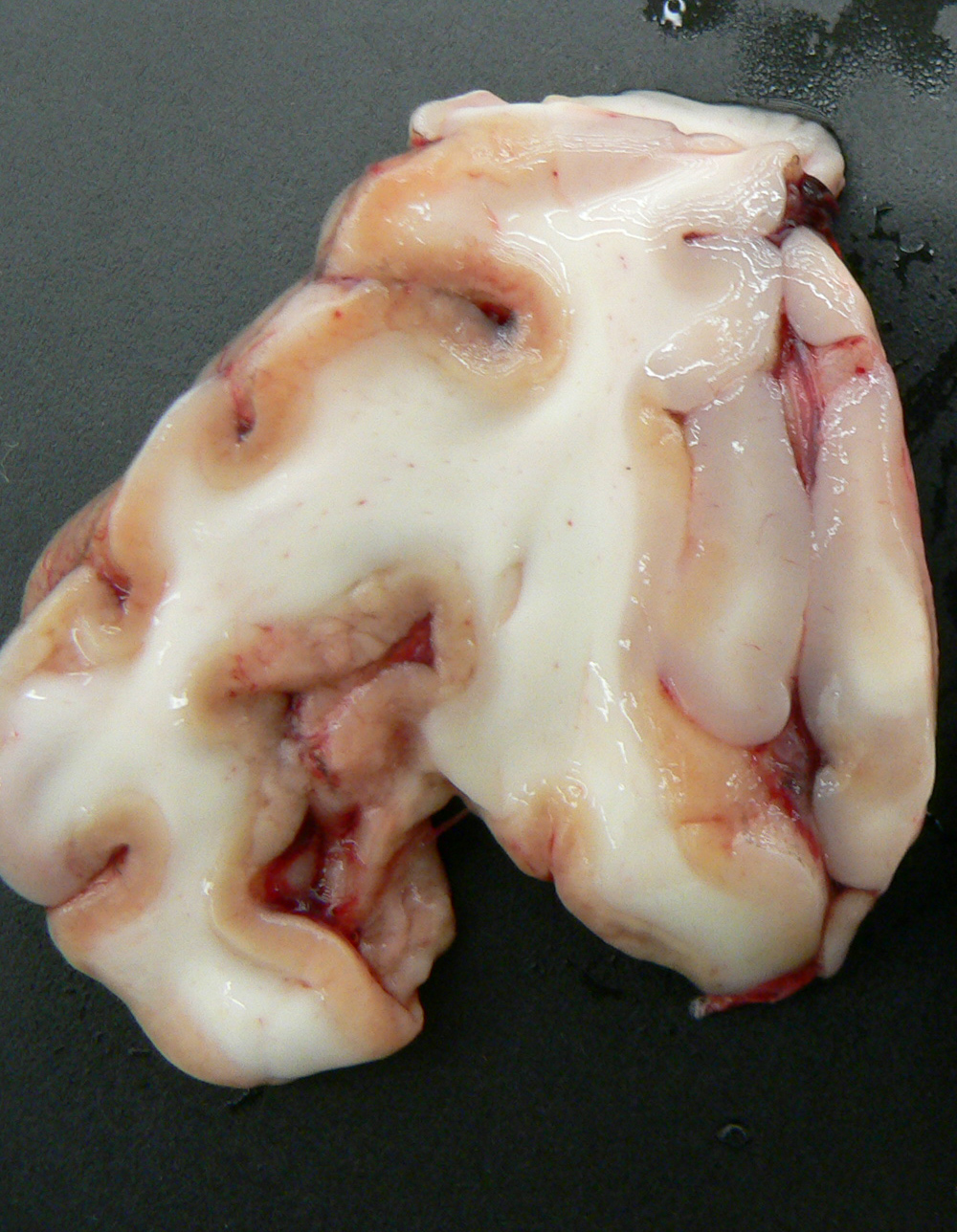
Gross Description:
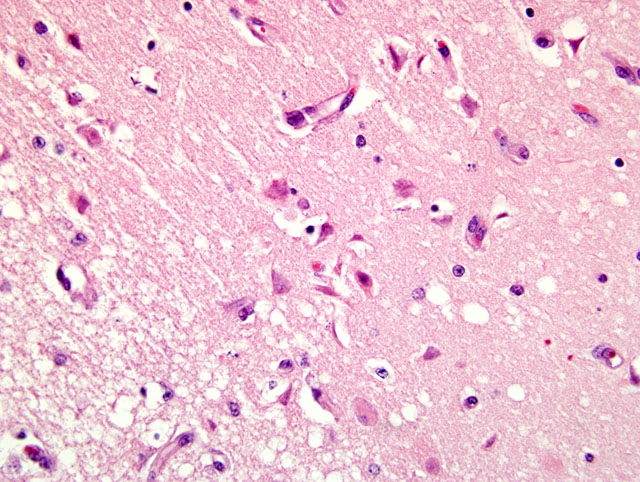
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The basis of sulfur-associated PEM in ruminants appears to be the excessive production of ruminal sulfide caused by the ruminal microbial reduction of ingested sulfur. Soluble hydrosulfide anion is in the rumen fluid phase, and hydrogen sulfide gas accumulates in the rumen gas cap. Non-reduced forms of sulfur (sulfate and elemental sulfur) are relatively nontoxic, while hydrogen sulfide and its ionic forms are highly toxic substances that interfere with cellular energy metabolism.(1) Sulfide inhibits cellular respiration by blocking cytochrome C oxidase in the electron transport chain. It is likely that sulfide produced in the rumen is absorbed into the blood and is capable of inhibiting energy metabolism of neurons and that this either directly causes neuronal necrosis or leads to necrosis by interfering with local cerebral blood flow and creating regional ischemia.(3) The most important route of sulfide absorption is unknown, but potentially occurs via the gastrointestinal mucosa or pulmonary mucosa after inhalation of eructated ruminal gas. The role of thiamine in these cases is not completely clear; however most current evidence indicates that sulfur-associated PEM does not involve reduced concentrations of thiamine in blood/tissues or reduced activity of transketolase, a thiamine-dependent enzyme.(3) A significant portion of PEM cases in ruminants may in fact be sulfur-associated rather than thiamine-associated, as was once thought.
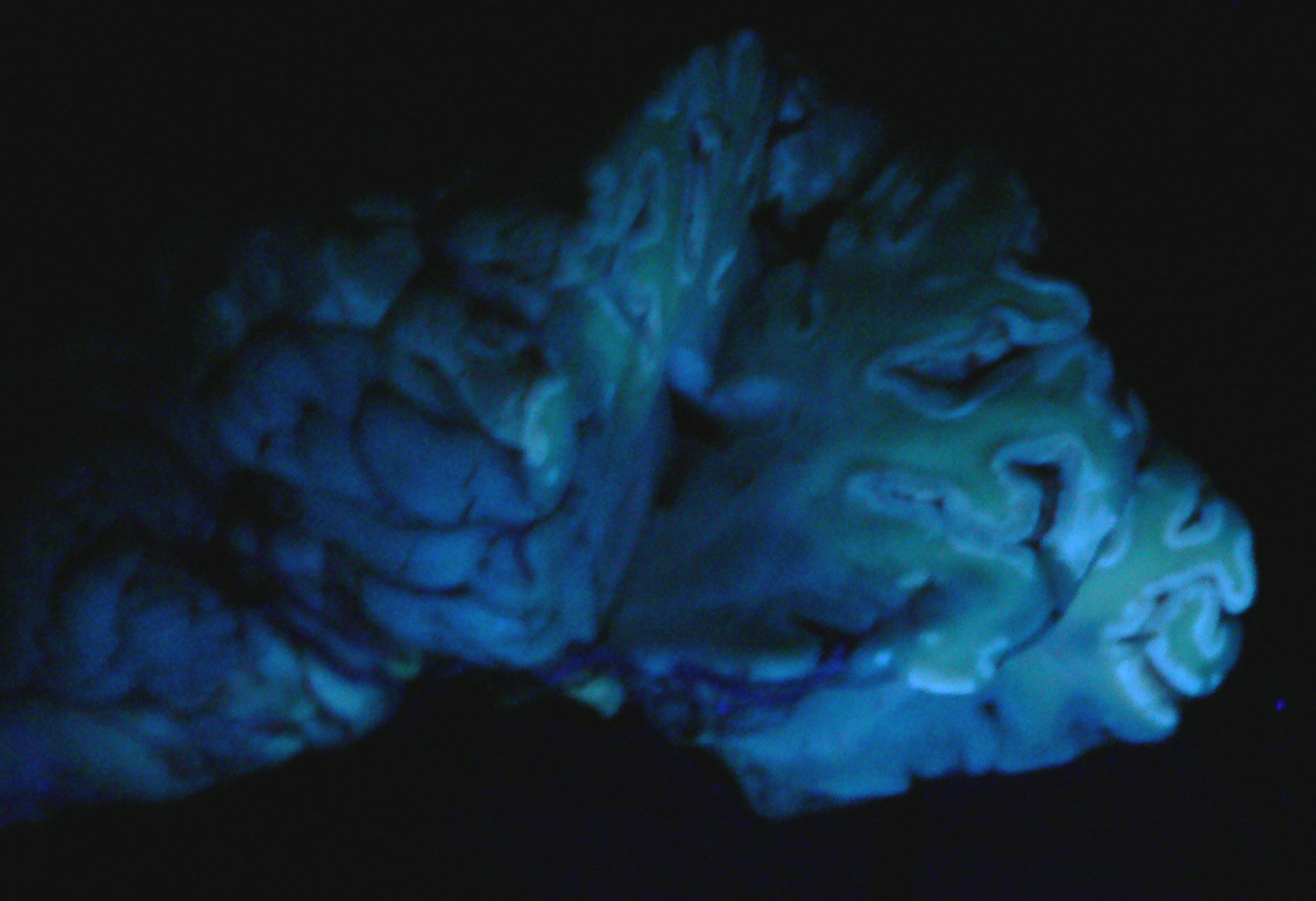
Exposure to large amounts of sulfur can occur from a variety of sources including both feed and water. The occurrence of PEM in ruminants associated with high dietary sulfur intake has been recognized with increasing frequency, particularly in Midwestern United States. This appears to be associated with the recent expansion of the fuel ethanol industry, as byproducts of corn (maize)-to-fuel ethanol production are being used with increasing frequency in ruminant diets. Ethanol byproducts are attractive feed ingredients due to their nutritional (especially protein) value and they are readily available at reduced cost (compared to corn) in Midwestern states. Sulfuric acid is utilized in ethanol byproduct production, and byproducts thus often contain significantly elevated levels of sulfur which represent a major source of dietary sulfur for these animals. In addition, there is much variation in the sulfur content of ethanol byproducts both within and between plants, and for this reason periodic monitoring is recommended for producers utilizing these products in rations.(2) In this case, the history of corn gluten meal feeding (a commonly fed ethanol byproduct) is considered to be the likely source of elevated sulfur, though feed samples were not evaluated for confirmation. This case represents a classic case of ruminant PEM both clinically and pathologically. Sulfur-related PEM manifests clinically as acute blindness, recumbency, seizures and death or a more subacute form characterized by visual impairment and ataxia. Lesions are classic and are characterized by extensive necrosis of cerebrocortical neurons. Grossly, there is fragmentation, malacia and loss of cortical gray matter that in some cases autofluoresces brightly when exposed to 366-nm UV light. At later stages, affected tissue becomes cavitated as macrophages infiltrate and necrotic tissue is removed.(1)
JPC Diagnosis:
Conference Comment:
Thiamine, or vitamin B1, is an essential dietary need in carnivores. Ruminants are able to produce their own thiamine from the diet via microbial production in the rumen.(3) Thiamine deficiency in carnivores often results from eating a diet high in fish containing thiamine-splitting enzymes (thiaminases). Sulfur dioxide, often used to preserve that fresh fish appearance, can destroy thiamine after it is metabolized into sulfates.(3)
If the disease is recognized early in its progression, thiamine supplementation can reverse the course of the disease. If clinical signs are present for several days, and a point of no return is passed, death is the result. Microscopic lesions include vacuolation of the neuropil, vascular dilation, hemorrhage, and necrosis. The periventricular gray matter is highly susceptible and the lesion is almost always bilateral and symmetrical most often involving the inferior colliculi.(3)
References:
2. Gould, D: Update on sulfur-related polioencephalomalacia. In: The Veterinary Clinics of North America: Food Animal Practice: Toxicology, eds. Osweiler GD and Galey FD. pp 481-496. 16(3): November 2000
3. Maxie MG, Youssef S: Nervous system. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol 1, pp.351-356. Elsevier Limited, Philadelphia, PA, 2007
4. McAllister, MM: Feed-Associated toxicants: sulfur. In: Clinical Veterinary Toxicology, ed. Plumlee K, pp 133-134. Mosby, St Louis, MO, USA, 2004