Signalment:
14-year-old, thoroughbred, gelding,
horse, (
Equus caballus).The patient was admitted for evaluation of chronic colic, and had
a distended abdomen on arrival. The horse was diagnosed with uroperitoneum and
was euthanized after a tear in the ventral portion of the urinary bladder was
confirmed on abdominal ultrasound and cystoscopy. Clinical signs of disease
involving other organ systems (
e.g. respiratory tract) were not
reported.
Gross Description:
The patient was in satisfactory nutritional and good postmortem
condition. The peritoneum contained abundant turbid, light-yellow fluid with a
distinct ammonia odor
(i.e. urine), and was hyperemic with adherent
plaques of fibrin. The urinary bladder was distended and a 7 x 5 cm thin,
gray-green and friable (necrotic) focus within the ventral wall was confirmed.
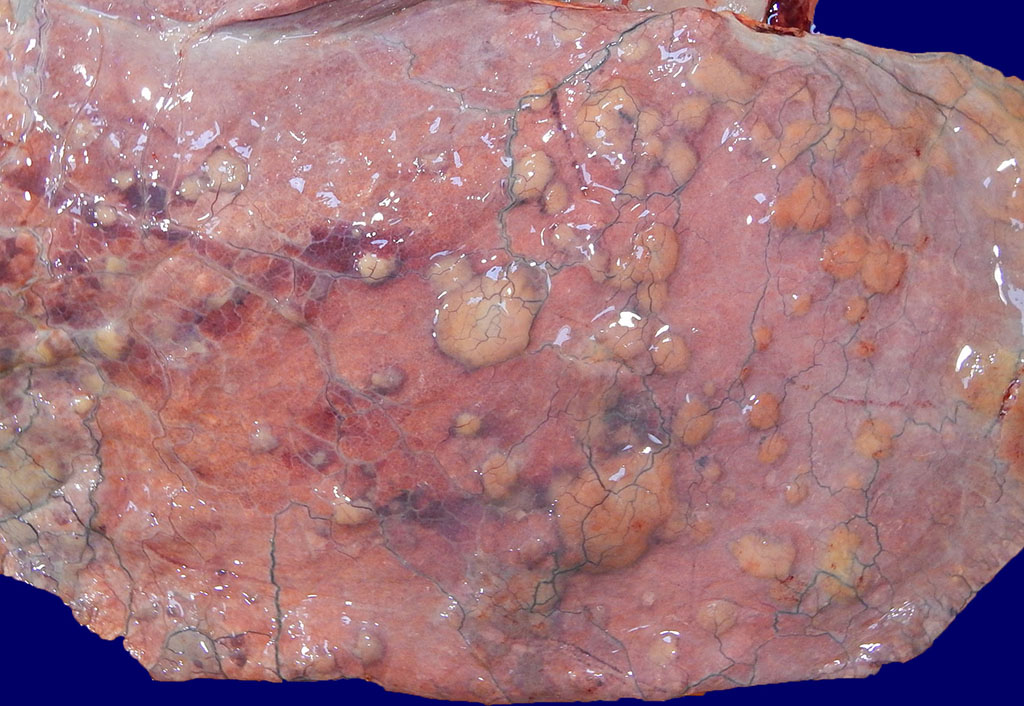
However, examination of the lungs revealed multifocal to coalescing 0.5- 5 cm
diameter firm tan nodules that extended into all fields, replacing the
pulmonary parenchyma and elevating the visceral pleura (figure 1). Intervening
pulmonary parenchyma was pale pink to dark red and mildly firm cranioventrally.
The brain, spinal cord, and remainder of the thoracic and abdominal viscera
were grossly unremarkable.
Histopathologic Description:
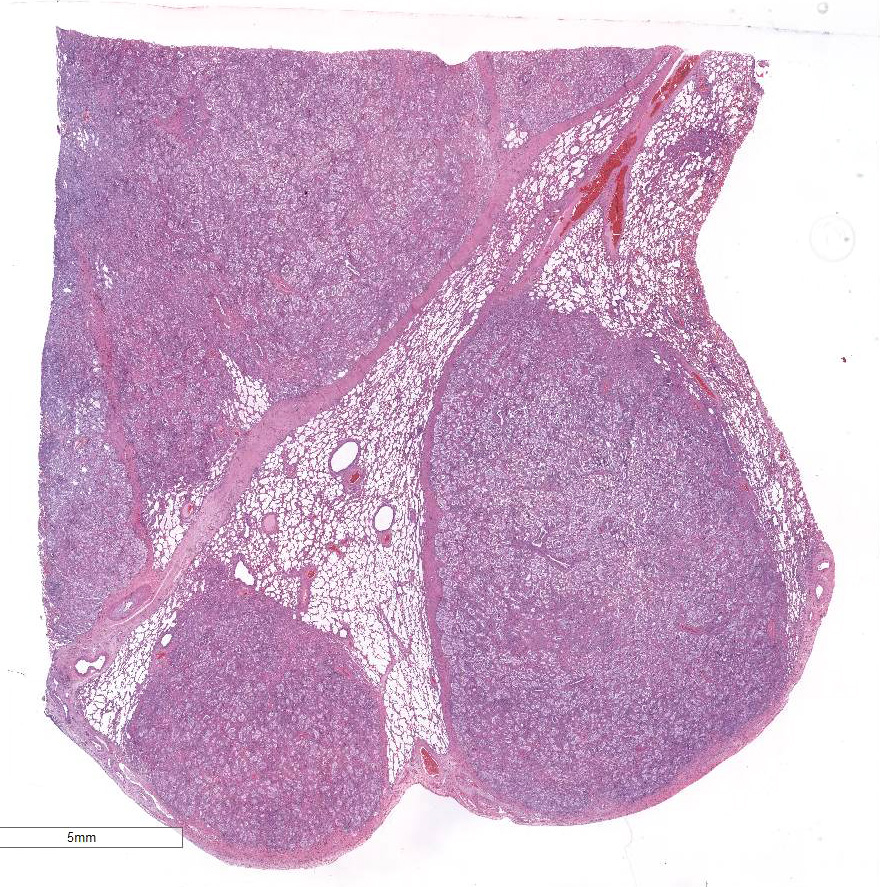
Approximately 80% of the pulmonary parenchyma was effaced by
multifocal to coalescing nodules composed of proliferating spindle cells
(fibroblasts) embedded within a fibrillar eosinophilic (collagenous) stroma,
and infiltrated by large numbers of lymphocytes and histiocytes, with fewer
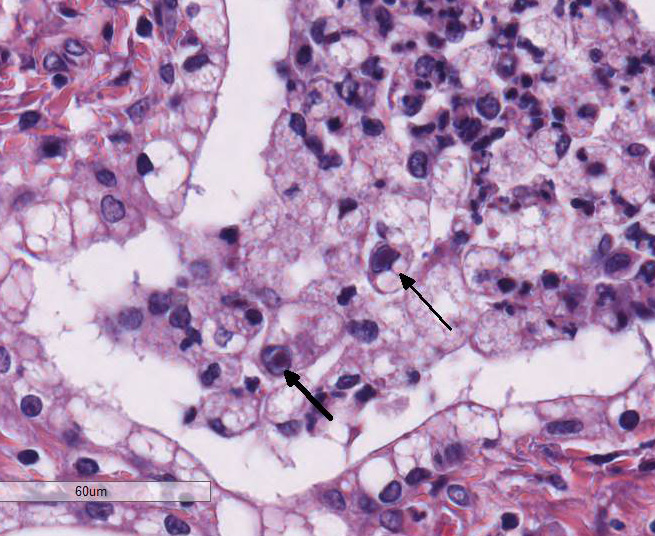
neutrophils. Occasionally, alveolar macrophages contained 3-4μm diameter
eosinophilic intranuclear inclusion bodies that peripheralized the chromatin
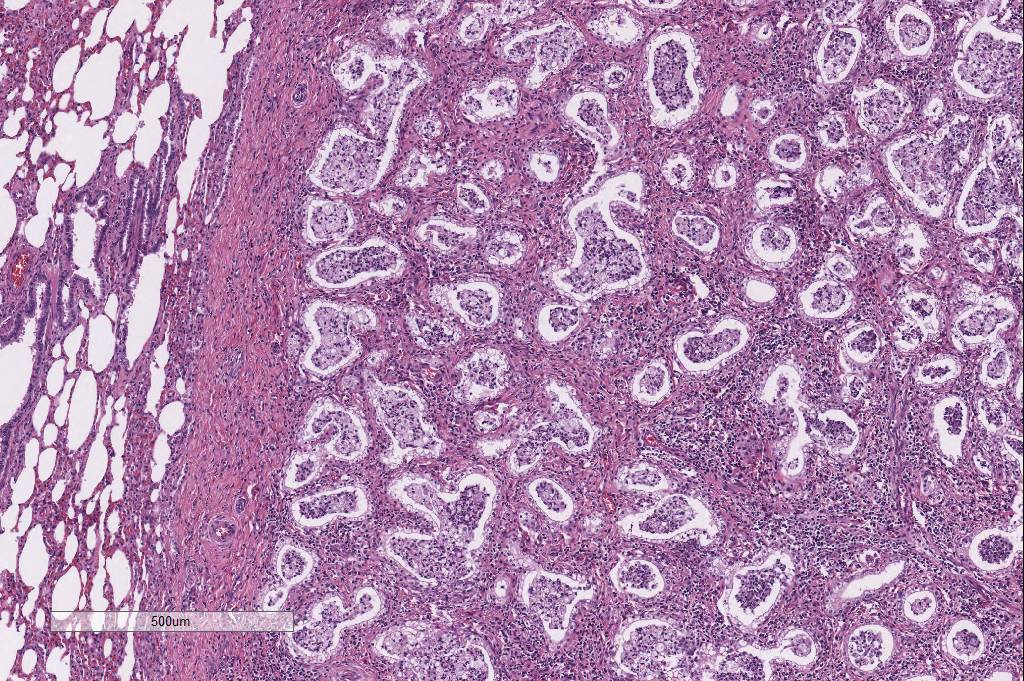
(Cowdry Type A inclusions; figure 2 (arrow)). Within affected regions, the
majority of alveoli were lined by plump cuboidal epithelial cells
(bronchiolization). Affected alveoli, and occasional bronchi and bronchioles,
were filled with numerous foamy macrophages, neutrophils, and cell debris.
Within unaffected regions of lung, alveoli were often ectatic (hyperinflated),
and there was mild multifocal alveolar hemorrhage.
Morphologic Diagnosis:
Lung: severe chronic
multifocalcoalescing sclerosing lymphohistiocytic and neutro-philic
bronchointerstitial pneumonia with intrahistiocytic intranuclear inclusion
bodies (consistent with equine multinodular pulmonary fibrosis).
Lab Results:
None.
Condition:
Equine multinodular pulmonary fibrosis, EHV-5
Contributor Comment:
Equine multinodular pulmonary fibrosis (EMPF), one of the
relatively few differentials for interstitial lung disease in the horse,
results in a unique gross and histologic appearance dominated by a nodular
pattern of marked interstitial fibrosis.
10 Clinical signs of
affected horses may include tachypnea, dyspnea, nasal discharge, coughing,
lethargy, inappetence, and poor body condition. Affected horses may have
increased bronchovesicular sounds, fever, and/or hypoxemia on clinical exam.
14
Hematology typically reveals a neutrophilia (in some cases with increased
bands), lymphopenia or lymphocytosis, and monocytosis.
7,14 A nodular
interstitial pattern is often identified on thoracic radiographs and
ultrasound, resulting in differential diagnoses of EMPF, fungal pneumonia and
pulmonary neoplasia. Concurrent with the interstitial pulmonary disease,
thoracic ultrasonography may also reveal pulmonary artery dilation, consistent
with pulmonary hypertension. Transtracheal wash fluid is predominantly
neutrophilic (degenerate or non-degenerate), with fewer macrophages.
14
No breed or sex predilection has been established, and although affected horses
are typically middle-aged or geriatric, cases have been reported in horses as
young as four years-old.
12
Two gross patterns of EMPF have been described, both of which are
characterized by multifocal moderately firm, tan, bulging nodules throughout
the pulmonary parenchyma. In the more common of the two gross forms (the form
exhibited by this horse), these nodules are numerous but small (up to 5 cm in
diameter), and coalesce, often resulting in scant intervening parenchyma. In
the second gross pattern, the nodules are less frequent, larger (up to 10 cm in
diameter), and discrete, with the intervening pulmonary parenchyma largely
unaffected.
12 Histologically, both gross forms are characterized by
extensive interstitial deposition of mature collagen, accompanied by moderate
mixed inflammatory infiltrates, consisting pre-dominantly of lymphocytes,
macrophages and neutrophils. Alveoli in affected regions are typically lined by
plump, cuboidal epithelium, and airways contain abundant neutrophils and
macrophages. Alveolar macrophages occasionally contain ampho-philic to
eosinophilic intranuclear inclusion bodies. Less frequently, the nodules
consist of dense sheets of disorganized collagen that completely efface the
normal alveolar pattern.
12
Equine
herpesvirus-5 (EHV-5) is a gamma herpesvirus strongly associated with EMPF, and
can be identified by polymerase chain reaction (PCR), immunohistochemistry
(IHC), in situ hybridization, virus isolation, and transmission electron
microscopy (TEM).
2,7,12-14 Although causality has not been proven by
meeting all of Kochs postulates, the main histologic and immunohistochemical
features have been recapitulated in horses inoculated with EHV-5 isolated from
EMPF-affected horses.
13 However, because inoculated horses lacked
clinical signs associated with EMPF, were PCR-negative for the inoculated EHV-5
viruses (within the lungs), and the virus was unable to be re-isolated, the
authors suggest that the immuno-histochemical
detection of virus in the inoculated horses having deposition of fibrous
connective tissue likely represented a pre-clinical, yet latent, phase of viral
infection.
13 Further supporting EHV-5 as an important etiologic
factor in the development of EMPF, quantitative real-time PCR performed in an
affected horse revealed higher viral load within the lungs than in other
tissues, with the highest load within the most severely affected/fibrotic
regions of lung.
4 In addition, gamma herpesviruses have been
associated with fibrotic lung diseases in other species, including a variably
fibrotic broncho-interstitial pneumonia with syncytial cell formation in
donkeys associated with asinine herpesvirus-4 and 5 (AHV-4 and 5),
3
and human idiopathic pulmonary fibrosis associated with human herpesvirus-8
(HHV-8) and Epstein-Barr virus.
10,11 Moreover, mice that are
knockouts for the IFNγ cytokine receptor (an important mediator of the Th1
antiviral response), develop progressive pulmonary interstitial fibrosis after
chronic infection with murine gamma herpesvirus-68.
5,11 Although the
precise mechanisms of gamma herpesvirus-induced fibrosis and immune system
evasion in most species are unknown, they are likely related to the specific
viral-induced cytokine, chemokine, and/or receptor profile within the host, which either directs the immune system toward a Th2
response (thereby inhibiting a Th1 response and facilitating fibrosis), or at
least prevents an effective Th1 antiviral response.
6,11 Such
mechanisms have already been established in the study of human gamma
herpesviruses, including viral CCL-1 (vCCL-1), vCCL-2, and vCCL-3 encoded by
HHV-8 that activate Th2 cell chemokine receptors (CCR8, CCR3 and CCR8, and
CCR4, respectively), and a viral IL-10 homologue produced by human Epstein-Barr
virus that results in inhibition of the Th1 antiviral response.
6,11
In spite of supporting evidence of EHV-5 infection inciting EMPF, EHV-5 may not
be the sole cause. As with many infectious diseases, concurrent viral
infections (such as EHV-2 and AHV-4 or 5), and the hosts immune status, may
also be important contributors to the pathogenesis of EMPF.
1,5,11,12
Although
successful treatment with corticosteroid and antiviral (acyclovir) therapy is
reported, overall, response to treatment is variable and the prognosis of this disease is generally considered poor.
14 This case
was unusual in that there were no reported clinical signs that were clearly
attributable to respiratory disease. However, a preclinical, latent phase of
infection is considered unlikely given the presence of numerous inclusion
bodies. Although the uroperitoneum accounted for the most recent episode of
colic, it is possible that the pneumonia caused chronic lethargy and
inappetence, vague signs that were interpreted as chronic colic. Regional trans-mural
necrosis of the urinary bladder was confirmed histologically, and was
attributed to pressure-induced ischemia secondary to distension. As there was
no evidence of urethral obstruction, a neurogenic cause was suspected. However,
no histologic lesions were identified within the spinal cord.
JPC Diagnosis:
Lung: Fibrosis, interstitial, nodular, multifocal to coalescing,
severe with lymphohistiocytic interstitial inflammation, alveolar neutrophilic
and histiocytic exudate, type II pneumocyte hyperplasia and histiocytic intranuclear
viral inclusion bodies, thoroughbred,
Equus caballus.
Conference Comment:
We
thank the contributor for both an excellent example and thorough summary of
equine multinodular pulmonary fibrosis (EMPF) in horses. This entity has also
been extensively reviewed in previous Wednesday Slide Conferences (
2011 Conference
1 Case 2
2012 Conference
24 Case 3
2013 Conference
18 Case 1
, but it was chosen again because of its highly distinctive and
unique histomorphology. Participants identified multifocal discrete lung
nodules with abundant interstitial fibrosis, marked type II pneumocyte
hyperplasia, and irregular alveolus-like spaces filled with an inflammatory
exudate composed of neutro-phils, fibrin, and alveolar macro-phages which
occasionally contain a 2-4 um magenta intranuclear inclusion body. Conference participants also noted especially prominent pleural
arteries in areas adjacent to the nodules of fibrosis with hypertrophic smooth
muscle in the tunica media indicative of pulmonary hypertension associated with
pulmonary fibrosis.
As mentioned by the
contributor, gamma herpesviruses have been associated with progressive
pulmonary fibrotic disorders in humans, donkeys, horses, and rodents. In dogs,
canine idiopathic pulmonary fibrosis is a progressive pulmonary fibrotic
disorder predominantly in aged West Highland white terriers (WHWT).
9
Recently, investigators have tried to elucidate a relation between this
disorder in WHWTs and gamma-herpesvirus infection; however, no evidence a
connection was found. Given this conditions predilection for WHWT, it is
thought that there is a genetic component to this disease in this breed of dog
rather than an infectious etiology.
9
In addition to pulmonary
fibrosis, conference participants discussed the association of a gamma
herpesvirus with retroperitoneal fibromatosis (RF), an aggressive proliferation
of highly vascular fibrous tissue subjacent to the peritoneum involving the
ileocecal junction and mesenteric lymph nodes in rhesus macaques.
10
RF is associated with co-infection of simian retrovirus 2 (SIV) causing simian
acquired immunodeficiency syndrome (SAIDS) and RF-associated herpesvirus
(RFHV). This condition is closely related to Kaposis sarcoma in humans, caused
by co-infection of human herpesvirus 8 (HHV8) and human immuno-deficiency virus
(HIV), and is one of the first illnesses associated with the development of
AIDS.
10 Additionally, rhesus macaque rhadinovirus, another
gammaherpesvirus, is also closely related to HHV8 and both have been associated
with the development of B-cell lymphoma.
8
References:
1. Back H, Kendall A, Grandón R, et al. Equine multinodular pulmonary
fibrosis in association with asinine herpesvirus type 5 and equine herpesvirus
type 5: a case report.
Acta Vet Scand. 2012;54(57):1-5.
2. Caswell JL, Williams KJ: Equine multinodular pulmonary fibrosis.
In: Maxie MG ed.
Jubb, Kennedy, and Palmers pathology of domestic animals. Vol
2. 6
th ed. St. Louis, Missouri: Elsevier; 2016:568-569.
3. Kleiboeker SB, Schommer SK, Johnson PJ, et al. Association of two
newly recognized herpesviruses with interstitial pneumonia in donkeys (
Equus
asinus).
J Vet Diagn Invest. 2002;14:273-280.
4. Marenzoni ML, Passamonti F, Lepri E, et al. Quantification of
Equid
herpesvirus 5 DNA in clinical and necropsy specimens collected from a horse
with equine multinodular pulmonary fibrosis.
J Vet Diagn Invest. 2011;23(4):802-806.
5. Mora AL, Woods CR, Garcia A, et al. Lung infection with
γ-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice.
Am
J Physiol Lung Cell Mol Physiol. 2005;289:L711-L721.
6. Nicholas J. Human gamma-herpesvirus cytokines and chemokine
receptors.
J Interf Cytok Res. 2005;25:373-383.
7. Niedermaier G, Poth T, Gehlen H. Clinical aspects of multinodular
pulmonary fibrosis in two warmblood horses.
Vet Rec. 2010;166:426-430.
8. Orzechowska BU, Powers MF, et al. Rhesus macaque
rhadinovirus-associated non-Hodgkin lymphoma: Animal model for KSHV associated
malignancies.
Blood. 2008; 112:4227-4234.
9. Roels E, Dourcy M, et al. No evidence of herpesvirus infection in
West Highland white terriers with canine idiopathic pulmonary fibrosis.
Vet
Pathol. 2016; 53(6):1210-1212.
10. Rose
TM, Strand KB, et al. Identification of two homologs of the Kaposis
sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal
fibromatosis in different macaque species.
J Virol. 1997; 71:4138-4144.
11. Tang
Y, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in
lungs of patients with idiopathic pulmonary fibrosis.
J Clin Microbiol.
2003;41(6):2633-2640.
12. Williams
KJ. Gammaherpesviruses and pulmonary fibrosis: evidence from humans, horses and
rodents.
Vet Pathol. 2014;51(2):372-384.
13. Williams
KJ, Maes R, Del Piero F, et al. Equine multinodular pulmonary fibrosis: a newly
recognized herpesvirus-associated fibrotic lung disease.
Vet Pathol. 2007;44:849-862.
14. Williams
KJ, Robinson NE, Lim A, et al. Experimental induction of pulmonary fibrosis in
horses with gammaherpesvirus Equine Herpesvirus 5.
PLoS ONE. 2013;8(10):e77754,
doi. 10.1371/journal.pone.0077754.
15. Wong
DM, Belgrave RL, Williams KJ, et al. Multinodular pulmonary fibrosis in five
horses.
J Am Vet Med Assoc. 2008;232(6):898-905.