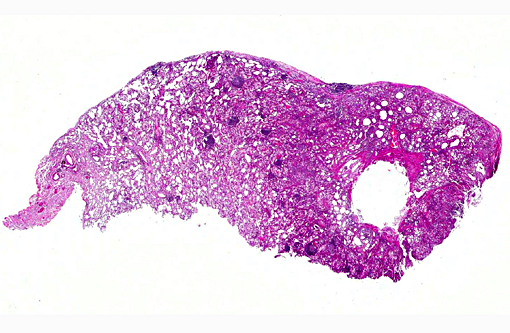
Signalment:
Gross Description:
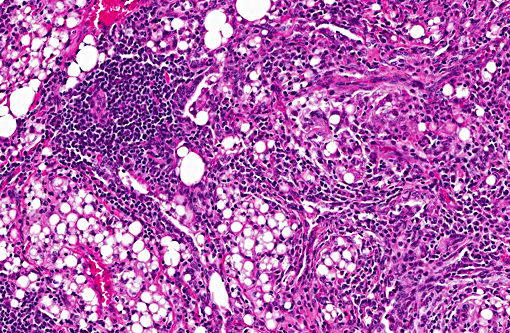
Histopathologic Description:
Multifocal to coalescing areas of varying sized infiltrates of macrophages and multinucleated giant cells engulfing and/or surrounding varying sized lipid droplets are observed primarily in alveolar airways. Many of these areas are also partially to completely surrounded by fibrous connective tissue with a mild to moderate lymphocytic and plasmacytic infiltrate. Extensive type II pneumocyte hyperplasia and multiple moderately-sized lymphoid aggregates/nodules are observed. On some slides is an abundance of necrotic mineralizing debris admixed with small amounts of fibrin within a large bronchiole that has denuded mucosal epithelium and focal areas of mild to moderate smooth muscle hypertrophy. Some slides have multifocal areas of pleural fibrosis which extends into the pulmonary parenchyma.
Morphologic Diagnosis:
Condition:
Contributor Comment:
There are two types of lipoid pneumonia: endogenous and exogenous. Endogenous lipoid pneumonia has been described in rats (20) dog ,(5, 19) genet,(18) cats ,(11) mongoose,(10) raccoons, (9) llama,(8) African grey parrot,(6) opossums, (3) Siberian tigers.(1) Exogenous lipoid pneumonia has been induced experimentally in mice and rats,(20) and found in two cases of horses(2,15) and a cow.(21) Lipoid pneumonia is an uncommon form of pneumonia.(7) It has been reported under different names, such as paraffinoma,(24) cholesterol pneumonia,(22) oil granulomas of the lung,(17) and lipid granulomatosis.
Exogenous lipoid pneumonia is more commonly reported in human literature(7) whereas it is the least reported in the veterinary literature.(11) It is caused by the inhalation or aspiration of lipid substances: animal fats, vegetable oils or mineral oil. Animal fat/oils elicit a very active inflammatory response. Mineral oils are fairly inert because they have no fatty acids and are rapidly emulsified and consumed by pulmonary macrophages. Vegetable oils are emulsified and not hydrolyzed by the lung lipase.(13) Aspiration may be due to age, psychiatric disorders, loss of consciousness ,(14) abnormality of deglutination (pharyngeal or esophageal) as well as gastroesophageal reflux(11,22) (oil floats on top of stomach fluids, thus oils may preferentially enter the airways).(14) Most occur when oils are used for medicinal purposes (constipation, oral health, nasal drops, etc).(12,16,17) A disproportionate population of fire breathers develop this type of pneumonia because of the liquid paraffin they use.(24)
Many lipid substances are non-irritating, can enter the tracheobronchial tree without stimulating a gag or cough reflex, and impair the mucociliary transport system. The pathophysiology is that of a chronic foreign body reaction. Once lipids are in alveoli, they are emulsified and taken up by macrophages. These cells cannot metabolize the fatty substances, so cells die, oil is released and rephagocytized. Over time, giant cell formation and fibrosis ensue. Diagnosis is made by the presence of large fat globules surrounded by macrophages and multinucleated giant cells as well as lipid-laden macrophages.(23)
Endogenous lipoid pneumonia, also called cholesterol or golden (accumulation of lipid in alveoli causing a yellow discoloration to the lungs) pneumonia, usually develop when lipids that normally reside in the lung tissue, most commonly cholesterol and its esters, escape from destroyed alveolar cell membranes (surfactant) distal to an obstructing airway lesion or damaged by an inflammatory process. It can also occur with fat emboli to the lung, pulmonary alveolar proteinosis, and lipid storage disorders (Niemann-Pick disease).(7) The pathogenesis is complex and may be related to retained epithelial secretions, cell breakdown, leakage from vessels, prolonged hypoxia, oxygen and carbon dioxide tension. It may be the result of transbronchial dissemination of breakdown products of cancer cells and secretions including mucin. Another though involves anoxic tissue injury stimulating phospholipases and mono-oxygenases, which in turn cause modification of low-density lipoprotein cholesterol. This cholesterol enhances lipid uptake by alveolar macrophages. Definitive diagnosis is demonstrating lipid-laden macrophages and cholesterol crystals.(7)
JPC Diagnosis:
Conference Comment:
Conference participants described the lung as approximately 75% affected by a predominantly granulomatous infiltrate focused on variably sized lipid vacuoles within alveolar lumina. Other prominent features include extensive type II pneumocyte hyperplasia, fibrosis, and multifocal hyaline membrane formation. Conference participants also noted the multifocal nodular lymphoid aggregates which led to a brief discussion on their histiogenesis (i.e. a chronic inflammatory response versus hyperplasia of preexisting bronchus/bronchiolar associated lymphoid tissue (BALT). The lipid expands the interstitium and prominently fills alveoli depending on location; in some areas, the precise location of the lipid, is difficult to determine due to fibrosis and inflammation. Additional histochemical stains used to highlight lipid include Oil Red O and Sudan black.
References:
1. Bolo E, Scaglione FE, Chiappino L, etal. Endogenous lipid (cholesterol) pneumonia in three captive Siberian tigers (Panthera tigris altaica). J Vet Diag Invest. 2012; 24:618-620.
2. Bos M, deBosschere H, Deprez P, etal. Chemical identification of the (causative) lipids in a case of exogenous lipoid pneumonia in a horse. Eq Vet J. 2002; 34:744-747.
3. Brown CC. Endogenous lipid pneumonia in opossums from Louisiana. J Wildl Dis. 1998; 24:214-219.
4. Carminato A, Vascellari M, Zotti A, et al. Imaging of exogenous lipoid pneumonia simulating lung malignancy in a dog. Can Vet J. 2011; 52:310-312.
5. Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 2. St. Louis, MO: Elsevier; 2015:517.
6. Costa T, Grifols J, Perpinan D. Endogenous lipid pneumonia in an African Grey Parrot (Psittacus erithacus erithacus). J Comp Pathol. 2013; 149:381-383.
7. Hadda V, Khilnani GC. Lipoid pneumonia: an overview. Expert Rev resp Med. 2010; 4:799-807.
8. Hamir AN, Andreasen CB, Pearson EG. Endogenous lipid pneumonia (alveolar histiocytosis) and hydrocephalus in an adult llama (Llama glama). Vet Rec. 1997; 141:474-475.
9. Hamir AN, Hanlon CA, Rupprecht CE. Endogenous lipid pneumonia (multifocal alveolar histiocytosis) in raccoons (Procyon lotor). J Vet Diagn Invest. 1996; 8:267-269.
10. Hayashi T, Stemmermann GN. Lipd pneumonia in the Hawaiian feral mongoose. J Pathol. 1972; 108:205-210.
11. Jones DJ, Norris CR, Samii VF, Griffey SM. Endogenous lipid pneumonia in cats: 24 cases (1985-1998). JAVMA; 216:1437-1440.
12. Kim JY, Jung JW, Choi JC, et al. Recurrent lipoid pneumonia associated with oil pulling. Int J Tuberculosis Lung Dis. 2014; 18:251-252.
13. Laurent F, Philippe JC, Vergier B, et al. Exogenous lipoid pneumonia: HRCT, MR, and pathologic findings. Eur Radiol. 1999; 9:1190-1196.
14. Marchiori E, Zanetti G, Mano CM, Hochhegger B. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Resp Med. 2011; 105:659-666.
15. Metcalfe L, Cummins C, Maischberger E, Katz L. Iatrogenic lipoid pneumonia in an adult horse. Ir Vet J. 2010; 63:303-306.
16. Moreau E, Rerolle C, Deveaux M, et al. Exogenous lipoid pneumonia as a contributory factor in a drug related death. J Forensic Sci. 2015; 60:514-517.
17. Papla B, Urbanczyk K, Gil T, et al. Exogenous lipoid pneumonia (oil granulomas of the lung). Pol J Pathol. 2011; 4:269-273.
18. Perpinan D, Steffen D, Napier JE. Pulmonary adenocarcinoma and endogenous lipid pneumonia in a common genet (Genetta genetta). J Zoo Wildl Med. 2010; 41:710-712.
19. Raya AI, Marco F-D, Nunez A, Afonso JC, et al. Endogenous lipid pneumonia in a dog. J Comp Pathol. 2006; 135:153-155.
20. Romero F, Shah D, Duong M, et al. Chronic alcohol ingestion in rats alters lung metabolism, promotes lipid accumulation, and impairs alveolar macrophage functions. Am J Respir Cell Mol Biol. 2014; 51:840-849
21. Smith BL, Alley MR, McPherson WB. Lipid pneumonia in a cow. New Zeal Vet J. 1969; 17:65-67.
22. Spickard A, Hirschmann JV. Exogenous lipoid pneumonia. Arch Intern Med. 1994; 154:686-692.
23. Wang C-W, Colby TV. Histiocytic lesions and proliferations in the lung. Semin Diagn Pathol. 2007; 24:162-182.
24. Weinberg I, Fridlender ZG. Exogenous lipoid pneumonia caused by paraffin in an amateur fire breather. Occupational Med. 2010; 60:234-235.