Signalment:
Gross Description:
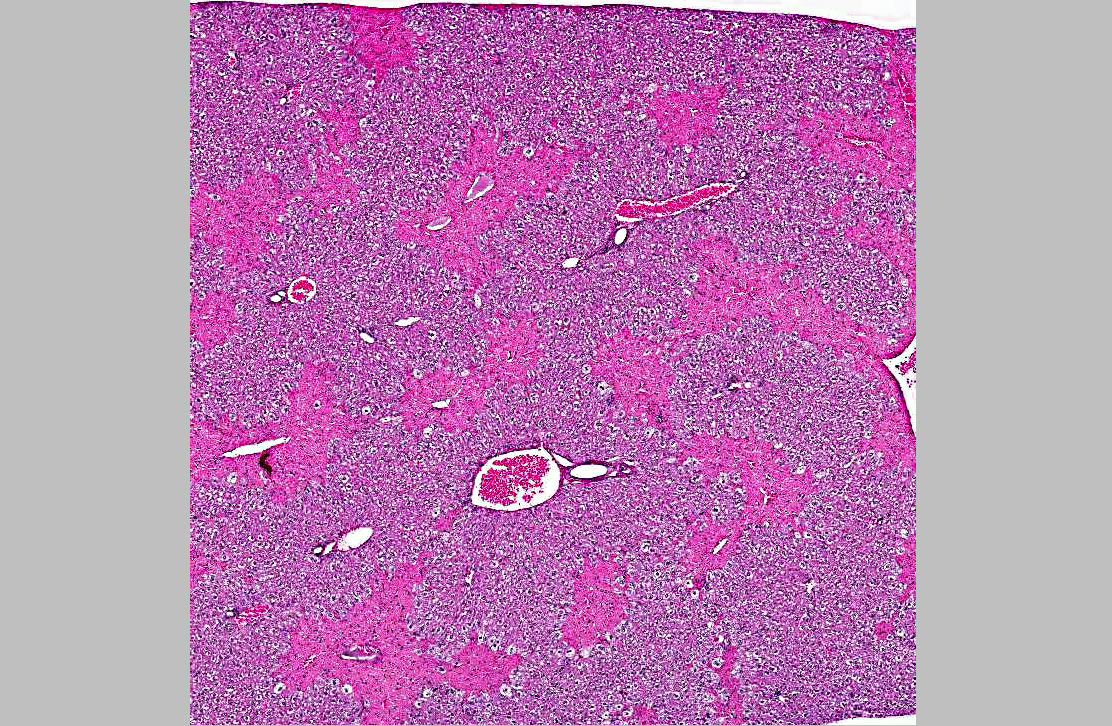
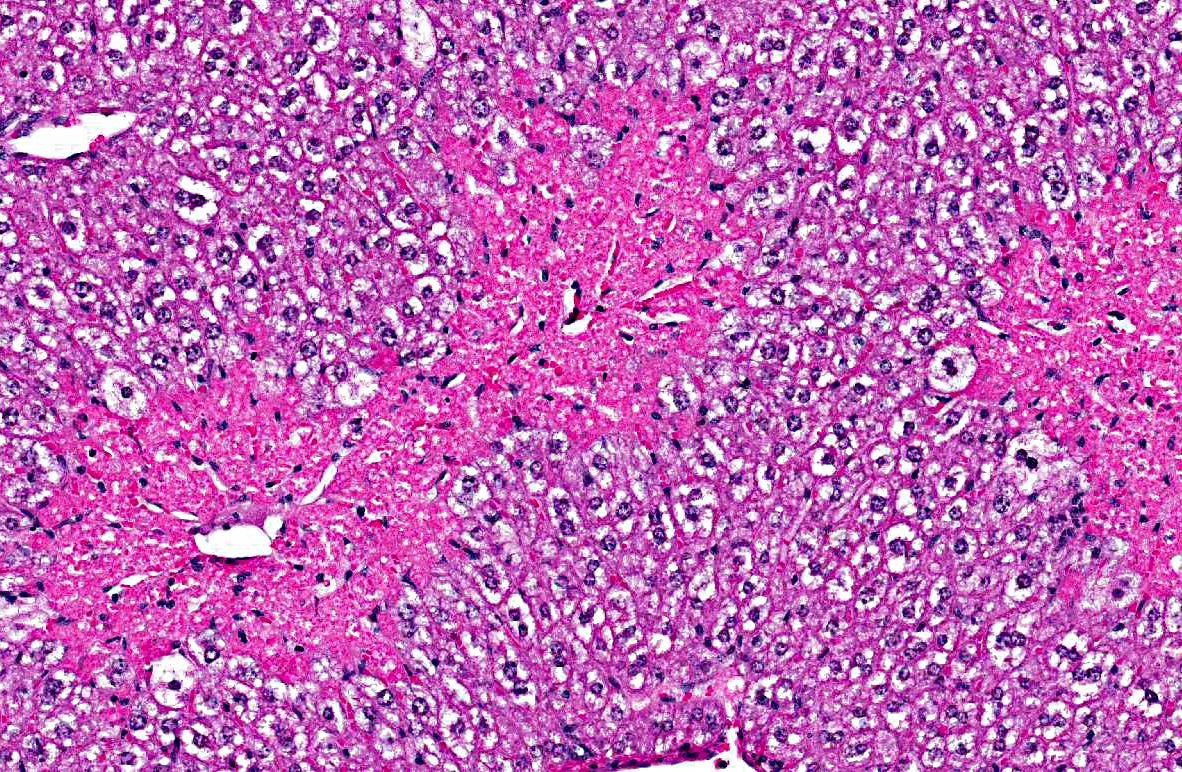
Histopathologic Description:
Morphologic Diagnosis:
1. Liver, centrilobular necrosis, diffuse, moderate, acute.
2. Liver, microvesicular hepatic lipidosis, diffuse, mild.
Condition:
Contributor Comment:
In most mammalian species (including humans, mice and dogs), the biotransformation of APAP involves conjugation with glucuronic acid or sulfate in the liver, which are subsequently excreted by urinary system.(1,2,3,4,7) A small amount of APAP is metabolized by the P-450 (CYP) system to the reactive metabolite N-acetyl pbenzoquinone imine (NAPQI) in centrilobular zone hepatocytes, which is subsequently scavenged by glutathione.(2,3,4,5,6)
APAP toxicity manifests primarily as centrilobular hepatic necrosis that can lead to acute liver failure. In humans, a single or accumulative daily toxic dose is usually >15-25 g.2,3,4 The mechanism of APAP hepatotoxicity is as follows:(2,3,5,6)
- At toxic doses, increased metabolism of APAP by the P-450 (CYP) system leads to higher concentrations of NAPQI formation in centrilobular zone hepatocytes.
- Higher concentrations of NAPQI deplete glutathione concentrations in centrilobular zone hepatocytes, leading to increased formation of reactive oxygen and nitrogen species.
- Increased oxidative stress in centrilobular zone hepatocytes results in alterations in calcium homeostasis and initiation of mitochondrial permeability transition.
- Loss of mitochondrial membrane potential in mitochondria of centrilobular zone hepatocytes leads to loss of ATP synthesis subsequent necrosis.
Cats are more sensitive to APAP toxicity and have a different pattern of APAP toxicity due to a species deficiency in glucuronyl transferase, resulting in relatively higher conversion rates of APAP to the reactive NAPQI metabolite.(1,7) In addition to depleting glutathione in centrilobular hepatocytes, NAPQI can also deplete glutathione in erythrocytes, leading to methemoglobinemia, Heinz body hemolytic anemia, and methemoglobinuria.(1,7)
Currently, APAP toxicity is the leading cause of liver failure in humans in the US and UK. Since APAP biotransformation and toxicity is similar between humans and mice, this makes mice an attractive and clinically relevant animal model for research into acetaminophen hepatotoxicity and other toxicities leading to centrilobular necrosis of the liver via the P-450 (CYP) system.(2,4)
JPC Diagnosis:
Conference Comment:
Drug metabolism occurs at several sites, including liver, intestines, lung, kidney, and plasma with the liver being the primary site. The liver contains a realtively large percentage of the bodys metabolizing enzymes, most importantly a group of proteins known as cytochrome P450 mixed function oxidases. These enzymes are found within microsomes in the smooth endoplasmic reticulum of centrilobular hepatocytes. The most common reaction catalyzed by the cytochrome P450 enzymes is a mono-oxygenase reaction in which a molecule of oxygen (O2) is split, with one oxygen atom oxidizing the xenobiotic and reduction of the other oxygen atom to produce a molecule of water. This oxidation-reduction reaction is responsible for the metabolism of a variety of drugs, to include paracetamol. Cytochrome P450 enzymes also catalyze reduction reactions to metabolize drugs such as prednisone, warfarin, and halothane. The third type of phase 1 metabolism reaction is hydrolysis, which is catalyzed by esterases and amidases (which also occur in hepatocytes and other extrahepatic sites, including plasma).(8)
Of the phase 2 reactions, glucuronidation and sulphation play a major role in the metabolism of paracetamol, with approximately 40% of the drug undergoing each reaction. As the contributor states, a small amount of paracetamol undergoes phase 1 Nhydroxylation which results in the toxic product Nacetyl-p-amino-benzoquinoneimine which is normally further metabolized (conjugated) by glutathione.(8)
Further discussion on this case centered on the use of severity modifiers (mild, moderate, severe) to quantify the extent of necrosis, which can be important in a case such as this in which there is a dose dependent response. Toxicologic pathologists often use such modifiers for quantification; however, this terminology is not traditionally used in diagnostic pathology at the AFIP/JPC, with the reasoning that necrosis in itself cannot be mild nor severe".
References:
2. Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369-405.
3. James LP, Mayeux PR, Hinson JA. Acetaminophen h e p a t o t o x i c i t y. Dr u g Me t a b Di s p o s i t i o n . 2003;31:1499-1506.
4. Jaschke H, McGill MR, Williams CD, et al. Current issues with acetaminophen hepatotoxicity a clinically relevant model to test the efficacy of natural products. Life Sciences. 2011;88:737-745.
5. Kumar V, Abbas AK, Fausto N. Cellular adaption, cell injury, and cell death. In: Kumar V, Abbas AK, Fausto N, eds. Robbins and Coltran Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005:25-26.
6. Maitra A, Kumar V. Environmental and nutritional pathology. In: Kumar V, Abbas AK, Fausto N, eds. Robbins and Coltran Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005:424.
7. Savides MC, Oehme FW, Nash SL, et al. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol Appl Pharmacol. 1984;74:26-34.
8. Schonborn JL, Gwinnutt C. The role of the liver in drug metabolism. Anesthesia tutorial of the week 179. ATOTW. 2010(179): 1-6. http://www.aagbi.org/sites/default/files/179-The-role-of-the-liver-in-drugmetabolism.pdf. Accessed 18 October 2012.