Signalment:
Gross Description:
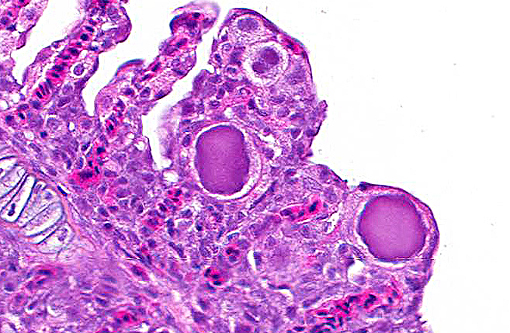
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Epitheliocystis is often considered an incidental finding.(1,5) However the prevalence, associated pathology and resultant mortality are highly variable among fish species and geographical locations. Risk factors associated with epitheliocystis infections include a higher morbidity and mortality in cultured fish, with losses up to 100%,(12) and seasonal variation related to water temperature.(12,13) Incidental epitheliocystis has been reported in Australian cultured barramundi.(1,11) A recent study of epitheliocystis in striped trumpeter Latris lineate, indicated a significant elevation of serum osmolality and lysozyme activity in infected fish, and this correlated also with the densities of infected cells in individuals. These findings indicate a measurable pathophysiological effect of epitheliocystis on the host. (10)
The etiological agents of epitheliocystis have not successfully been cultured in vitro, despite using a range of media and cell lines,(9) thus limiting our knowledge of this disease.(10,12) Molecular studies have identified a large variety of causative agents, however not all of these have included in situ methods for confirming the agents relation to the intracellular inclusions.(15) Agents identified by 16S rDNA include Candidatus Piscichlamydia salmonis in Atlantic salmon (Salmo salar)(4) and Candidatus Parilichlamydia carangidicola in Yellowtail kingfish (Seriola lalandi)(14), with sequences consistent with rRNA of Chlamydiales isolated from epitheliocystis in leafy seadragon (Phycodurus eques),(11) silver perch (Bidyanus bidyanus),(11) grass carp (Ctenopharyngodon idella)(8) and barramundi (Lates calcarifer)(11). Mixed infections occurring in the same fish species have also been reported.(13)
A review by Nowak and associates (2006) revealed the most reliable test for epitheliocystis is histology. In some cases it may also be seen grossly or on wet preparations, however these techniques are not as sensitive.(12)
JPC Diagnosis:
1. Gills: Lamellar epithelial hyperplasia and hypertrophy, with multifocal lamellar fusion and numerous coccobacilli.Â
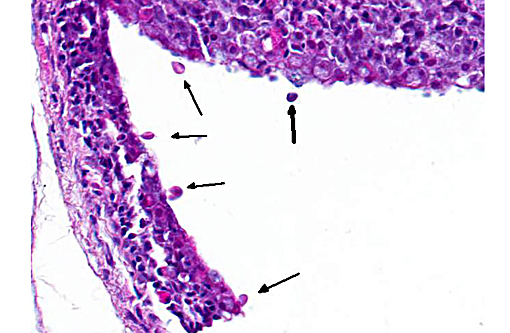
2. Skin, branchial cavity: Epithelial hyperplasia, diffuse, mild, with extracellular protozoans.Â
Conference Comment:
The specific cause of epitheliocystis remains elusive, and these bacterial colonies can often be observed without any other apparent pathology. A true causal relationship has not necessarily been demonstrated. Adding interest to this case, there are numerous flagellated protozoans along the skin surface and occasionally within the branchial cavity. These are most consistent with Ichthyobodo nectar, an important parasite of hatcheries which is capable of producing significant pathology of the skin, gills and fins. This parasite is found in both fresh water and marine species and have been known to induce T cell and IgT lymphocyte depletion in the skin under experimental conditions.(2) Whether its presence here is related to the gill pathology was a point of conjecture among conference participants and is left for speculation among our readers.Â
References:
1. Anderson, I.G., Prior, H.C. (1992) Subclinical epitheliocystis in barramundi, Lates catcarifer, reared in sea cages. Australian Veterinary Journal 69(9): 226-227
2. Buchmann K. Impact and control of protozoan parasites in maricultured fishes. Parasitology. 2015;142:168-177.
3. Camus, A., Soto, E., Berliner, A., Clauss, T., Sanchez, S. (2013) Epitheliocystis hyperinfection in captive spotted eagle rays Aetobatus narinari associated with a novel Chlamydiales 16S rDNA signature sequence. Diseases of Aquatic Organisms, 104: 1321
4. Draghi, A., Popov, V.L., Kahl, M.M., Stanton, J.B., Brown, C.C., Tsongalis, G.J., West, A.B., Frasca, S. (2004) Characterization of Candidatus Piscichlamydia salmonis (Order Chlamydiales), a Chlamydia-Like Bacterium Associated With Epitheliocystis in Farmed Atlantic Salmon (Salmo salar). Journal of Clinical Microbiology, 42 (11) 52865297
5. Ferguson H.W., (Ed.) (2006) Systemic Pathology of Fish. Scotian Press, London, pg.53
6. Fryer, J.L., Lannan C.N. (1994) Rickettsial and Chlamydial Infections of Freshwater and Marine Fishes, Bivalves, and Crustaceans. Zoological Studies 33(2): 95-107.
7. Groff, J.M., LaPatra, S.E., Munn, R.J., Anderson, M.L., Osburn, B. I. (1996) Epitheliocystis infection in cultured white sturgeon (Acipenser transmontanus): antigenic and ultrastructural similarities of the causative agent to the chlamydiae, Journal of Veterinary Diagnostic Investigation, 8: 172-180
8. Hoffman G.I., Dunbar, C.E., Wolf, K., Zwillenberg, L.O. (1969) Epitheliocystis, a new infectious disease of bluegill (Lepomis macrochirus). Antonie van Leevenhoek Journal of Microbiology and Serology 35, 146-158.
9. Kumar, G., Mayrhofer, R., Soliman, H., El-Matbouli, M. (2013) Novel Chlamydiales associated with epitheliocystis in grass carp (Ctenopharyngodon idella). Veterinary Record 172: 47-49
10. Lai, C.C., Crosbie, P.B.B., Battaglene, S.C., Nowak, B.F. (2013) Effects of epitheliocystis on serum lysozyme activity and osmoregulation in cultured juvenile striped trumpeter, Latris lineata (Forster). Aquaculture 388391 99104
11. Meijer, A., Roholl, P.J.M., Ossewaarde, J.M., Jones, B., Nowak, B.F. (2006) Molecular Evidence for Association of Chlamydiales Bacteria with Epitheliocystis in Leafy Seadragon (Phycodurus eques), Silver Perch (Bidyanus bidyanus), and Barramundi (Lates calcarifer). Applied and Environmental Microbiology 72 (1) 284290
12. Nowak, B. F., LaPatra, S.E. (2006) Epitheliocystis in fish. Journal of fish diseases 2006 29, 573-588.
13. Schmidt-Posthaus, H., Polkinghorne, A., Nufer, L., Schifferli, A., Zimmermann, D.R., Segner, H., Steiner, P., Vaughan, L. (2012) A natural freshwater origin for two chlamydial species, Candidatus Piscichlamydia salmonis and Candidatus Clavochlamydia salmonicola, causing mixed infections in wild brown trout (Salmo trutta). Environmental Microbiology 14(8), 20482057
14. Stride, M.C., Polkinghorne, A., Miller, T.L., Groff, J.M., LaPatra, S.E., Nowak, B. F. (2013) Molecular Characterization of Candidatus Parilichlamydia carangidicola, a Novel Chlamydia-Like Epitheliocystis Agent in Yellowtail Kingfish, Seriola lalandi (Valenciennes), and the Proposal of a New Family, Candidatus Parilichlamydiaceae fam. nov. (Order Chlamydiales). Applied and Environmental Microbiology 79 (5)15901597
15. Toenshoff E. R., Kvellestad, A., Mitchell, S.O., Steinum, T., Falk, K., Colquhoun D.J., Horn, M. (2013) A Novel Betaproteobacterial Agent of Gill Epitheliocystis in Seawater Farmed Atlantic Salmon (Salmo salar). PLoS ONE 7(3): e32696. doi:10.1371/journal.pone.0032696