Signalment:
Gross Description:
- Moderate multifocal dorsal and lateral alopecia with mild seborrhoea and exudative dermatitis
- Severe trauma to the head with comminuted fractures of the skull and jaw (as per method of euthanasia)
- Poor body condition
- Duodenal cestodiasis
- Colonic helminthiasis
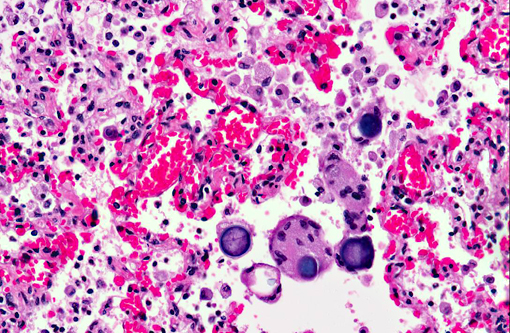
Histopathologic Description:
Morphologic Diagnosis:
1. Lung: Moderate histiocytic interstitial pneumonia and fibrosis with intralesional fungal elements (interpreted as Emmonsia parva).
2. Lung: Multifocal alveolar hemorrhage and congestion.
Condition:
Contributor Comment:
Pulmonary adiaspiromycosis is known primarily to occur in small rodents, carnivores and mustelids. In Australia, E. parva is described as the cause of pulmonary adiaspiromycosis in wombats on the basis of fungal morphology, although results of genetic characterization and confirmation of organism identity is yet to be reported.(11,12) In New Zealand, E. crescens is the reported cause of pulmonary adiaspiromycosis in the brushtail possum (Trichosurus vulpecula),(9) most likely secondary to co-habitation of the opossum with introduced British mammals (otter, stoat, weasel, mole, red fox and pine martin) in which E. crescens is widespread in the UK.(3) Adiaspiromycosis in humans is rare; most human infections are attributed to E. crescens although E. parva may be observed in AIDs patients.(5,6) Rarely, fatal human infections have been described.(2)
Wombats are large herbivorous burrowing marsupials native to Australia, of which there are three extant species: the southern hairy nosed wombat (Lasiorhinus latifrons), the northern hairy nosed wombat (Lasiorhinus krefftii), and the common wombat (Vombatus ursinis). The southern hairy nosed wombat is native to South Australia and it is estimated that up to 100,000 remain in the wild. The wombat presented in this case was culled and examined as part of a larger study examining skin disease and poor body condition in wombats in the Murrayland region of South Australia. Pulmonary adiaspiromycosis was observed in all wild wombats culled concurrently from this site. Gross lung lesions were not evident at post mortem. Previously reported gross findings in affected wombats have ranged from minimal change, to pale consolidation of ventral lung lobes with mucopurulent exudate in the bronchi and bronchioles.(11,12) The significance of pulmonary adiaspiromycosis in these wombats is uncertain; however, it may have contributed to poor body condition. Alternatively pulmonary fungal load and infection may have been exacerbated due to the presence of concurrent disease or immune suppression. Investigations into Southern hairy nosed wombat health in the region are continuing.
Aleuriospores of Emmonsia are ubiquitous and soil borne, and on inhalation form thick-walled non-replicating adiaspores in host tissues which continue to increase in size. Infection of wombats is thought to occur when they are pouch young, and a linear increase in Emmonsia spherule size with increasing wombat age has been observed.(11) The habitat and burrowing habits of the wombat is thought to render them prone to infections.(10) Southern hairy nosed wombats spend up to three-quarters of their time underground, and have small home ranges centered around their clay/calcrete or calcrete warrens.(7)
Emmonsia adiaspores must be differentiated in tissue section from other dimorphic fungi forming yeasts in host tissues. Phylogenetic studies recently found isolates of E. parva to be closer to Blastomyces dermatitidis than E. crescens, and the authors further suggest that there may be little basis to maintain Blastomyces and Emmonsia as separate genera.(13) In tissue section, the yeasts may be distinguished as either budding yeasts (B. dermatitidis and H. capsulatum) or thick-walled, or non-budding adiaspores (Emmonsia). Emmonsia adiaspores also resemble Coccidoides immitis in tissue section, with the exception that Emmonsia lacks internal spores.(14)
JPC Diagnosis:
Conference Comment:
With Blastomyces dermatitidis being a close relative of Emmonsia spp., it is curious how dramatically different the extent of disease is between the two species. Emmonsia spp. is more typically self-limiting and often human patients receive only supportive therapy,(1) which provides a stark contrast to B. dermatitidis which incites a more dramatic granulomatous reaction and is capable of spreading systemically.(4) It is suspected that Emmonsia spp. lacks the virulence factors identified with B. dermatitidis and the other dimorphic fungi which are well known to cause significant respiratory and often systemic disease in animals.
References:
1. Anstead GM, Sutton DA, Graybill JR. Adiaspiromycosis causing respiratory failure and a review of human infections due to Emmonsia and Chrysosporium spp. J Clin Microbiol. 2012;50(4):1346-1354.
2. Barbas Filho JV, Amato MB, Deheinzelin D, Saldiva PH, de Carvalho CR: Respiratory failure caused by adiaspiromycosis. Chest 97: 1171-1175, 1990
3. Borman AM, Simpson VR, Palmer MD, Linton CJ, Johnson EM: Adiaspiromycosis due to Emmonsia crescens is widespread in native British mammals. Mycopathologia 168: 153-163, 2009
4. Caswell JL, Williams KJ. Respiratory system. In: Maxie, MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2007:641-642.
5. Echavarria E, Cano EL, Restrepo A: Disseminated adiaspiromycosis in a patient with AIDS. J Med Vet Mycol 31: 91-97, 1993
6. England DM, Hochholzer L: Adiaspiromycosis: an unusual fungal infection of the lung. Report of 11 cases. Am J Surg Pathol 17: 876-886, 1993
7. Finlayson GR, Shimmin GA, Temple-Smith PD, Handasyde KA, Taggart DA: Burrow use and ranging behaviour of the southern hairy-nosed wombat (Lasiorhinus latifrons) in the Murraylands, South Australia. Journal of Zoology 265: 189 - 200, 2005
8. Hubalek Z: Emmonsiosis of wild rodents and insectivores in Czechland. J Wildl Dis 35: 243-249, 1999
9. Johnstone AC, Hussein HM, Woodgyer A: Adiaspiromycosis in suspected cases of pulmonary tuberculosis in the common brushtail possum (Trichosurus vulpecula). N Z Vet J 41: 175-178, 1993
10. Ladds P: Pathology of Australian Native Wildlife, 1 ed. CSIRO Publishing, Melbourne, 2009
11. Mason RW, Gauhwin M: Adiaspiromycosis in south Australian hairy-nosed wombats (Lasiorhinus latifrons). J Wildl Dis 18: 3-8, 1982
12. Nimmo J, Krockenberger M, Smith EF: Pulmonary adiasporomycosis in a Victorian wombat. In: Australian Society of Veterinary Pathology Annual Meeting, pp. 65-67. Attwood, Victoria 2007
13. Peterson SW, Sigler L: Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J Clin Microbiol 36: 2918-2925, 1998
14. Sigler L: Agents of adiaspiromycosis. In: Topley & Wilsons microbiology and microbial infections, eds. Ajello LHay R, 9th ed., pp. 571-583. Arnold, London, 1999
15. Sigler L: Ajellomyces crescens sp. nov., taxonomy of Emmonsia spp., and relatedness with Blastomyces dermatitidis (teleomorph Ajellomyces dermatitidis). J Med Vet Mycol 34: 303-314, 1996