Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 5
Sept 26th, 2018
CASE II: S1408459 (JPC 4066395-00).
Signalment: An aborted Angus bull calf, Bos taurus, bovine
History: An aborted Angus bull calf of approximately 6 month gestation; second first time heifer to have aborted fetus on the previously non-grazed ranch.
Gross Pathology: A 10.56 kg Angus bull calf fetus with crown-to-rump length of 54 cm and in good post-mortem state. Lung lobes were diffusely red and samples sank in 10% formalin [non-inflated lung]. The liver weighed 0.84 kg, was deep red-brown, nodular, markedly enlarged with rounded margins, and had a cobblestone appearance with multiple, pinpoint white foci scattered throughout. Spleen and thymus were markedly enlarged and diffusely lymph nodes were enlarged and prominent.
No gross lesions / abnormalities were noted in other viscera, brain, umbilicus, cranial vault and in bones and joints of all limbs. Placenta was not submitted.
Laboratory results: Tissues tested negative for multiple agents on a full bovine abortion panel which included Leptospira sp., Salmonella sp., Campylobacter sp., Brucella sp., bovine herpes virus 1, and bovine viral diarrhea virus [BVDv]. Liver minerals including selenium were within acceptable concentrations.
Submitted dam serum had marginally low magnesium levels, and was negative for antibodies to Leptospira spp., BVDv-1, BVDv-2, BHV-1, Neospora caninum and Brucella abortus.
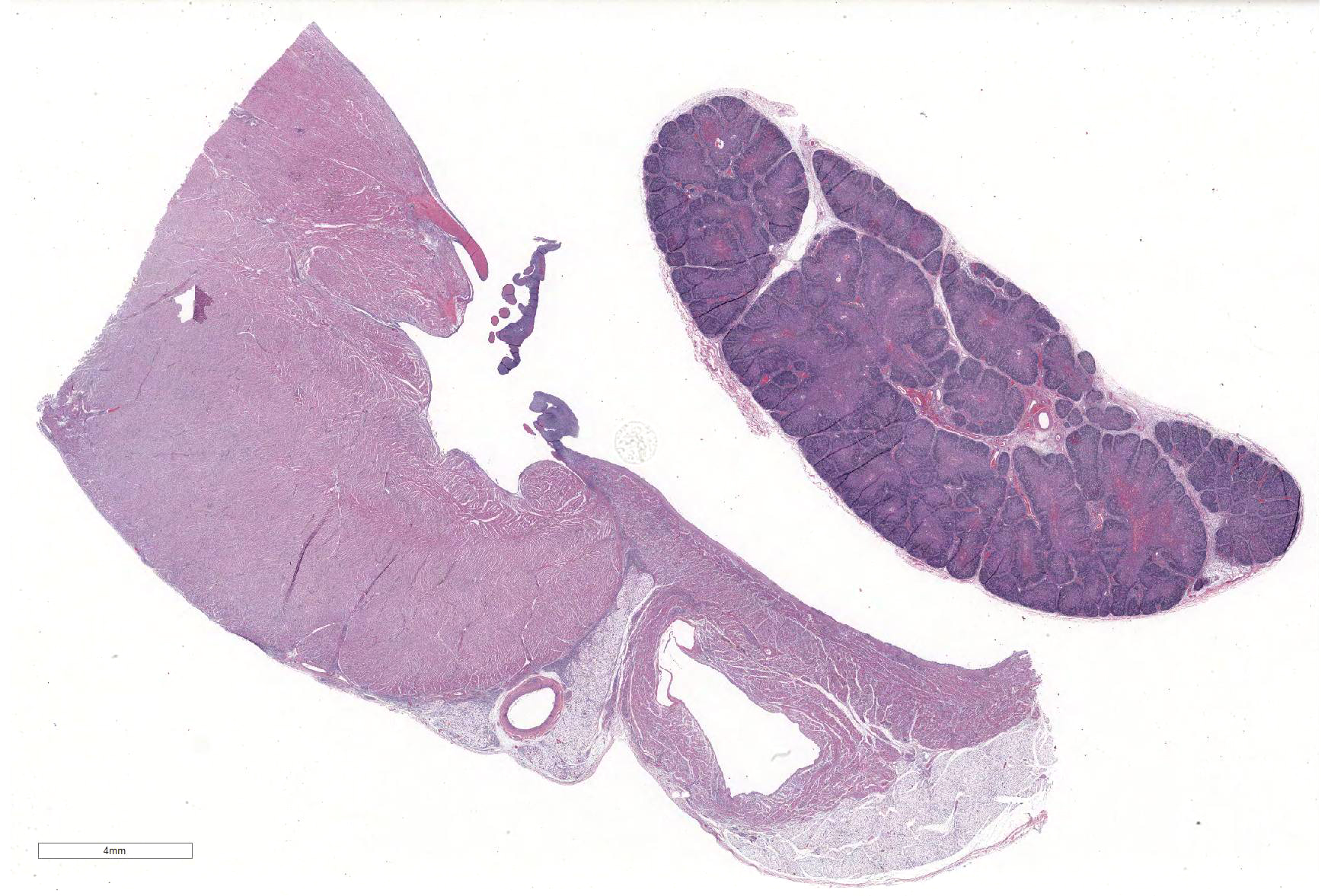
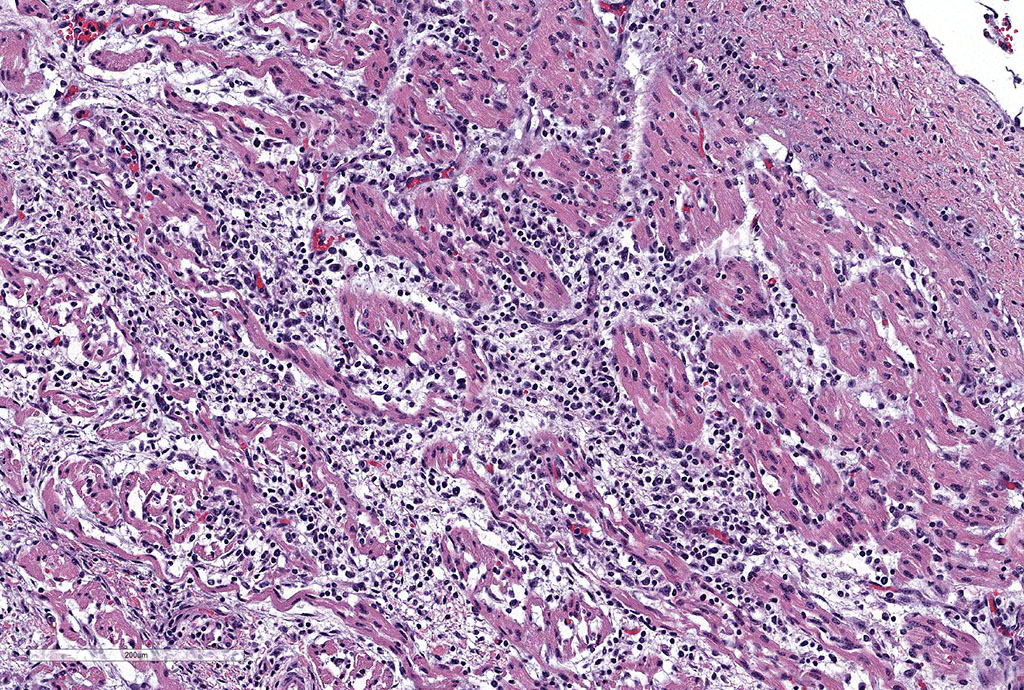
Microscopic Description: Slides contain a section each of thymus and heart.
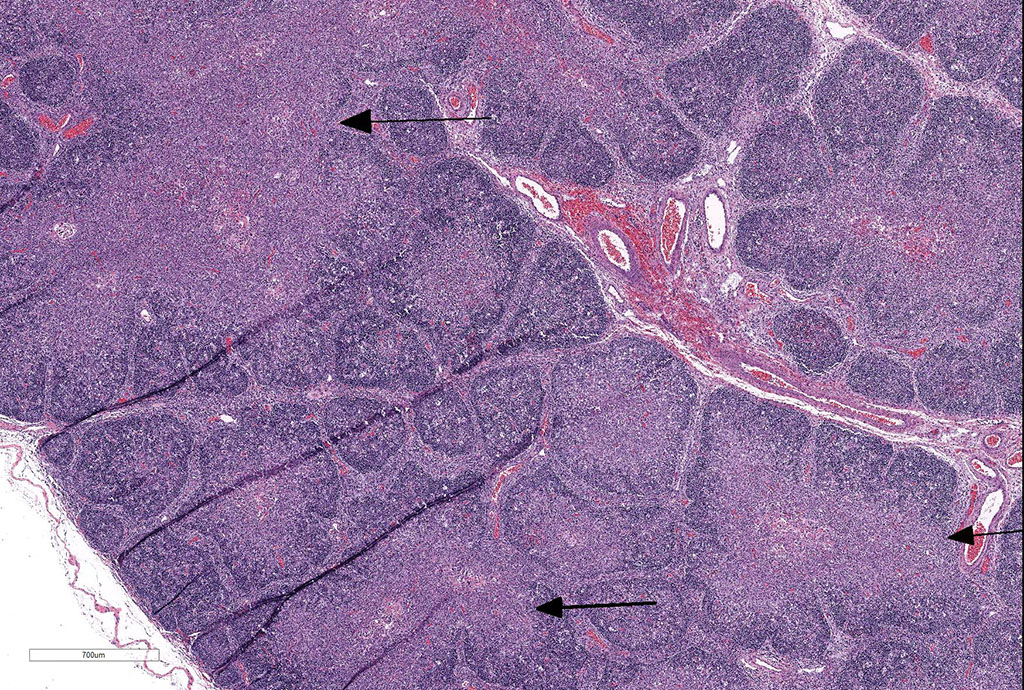
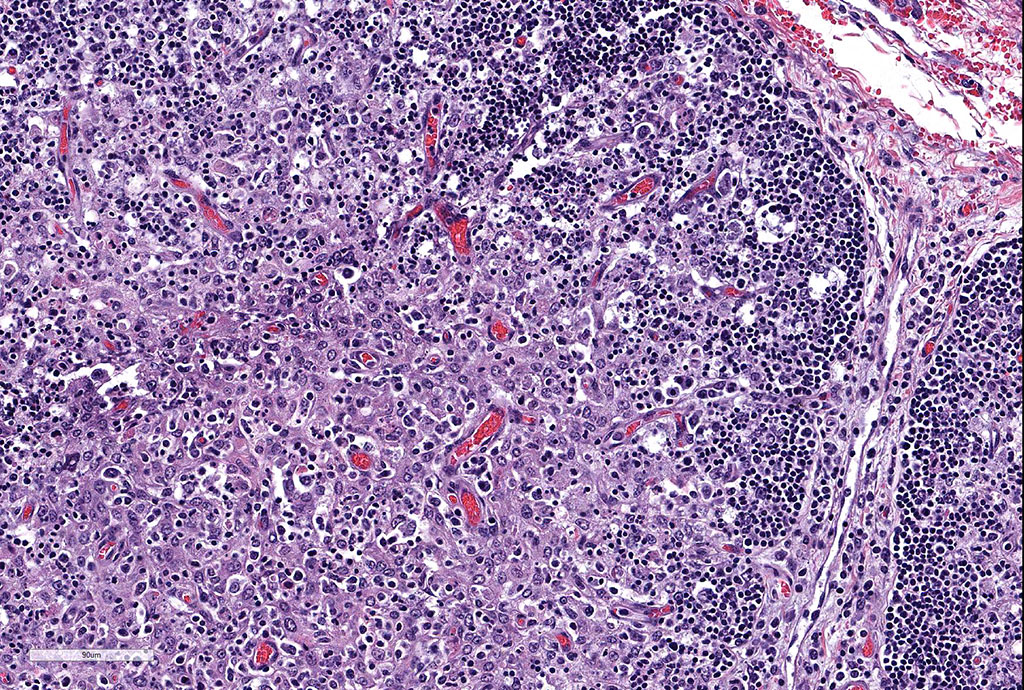
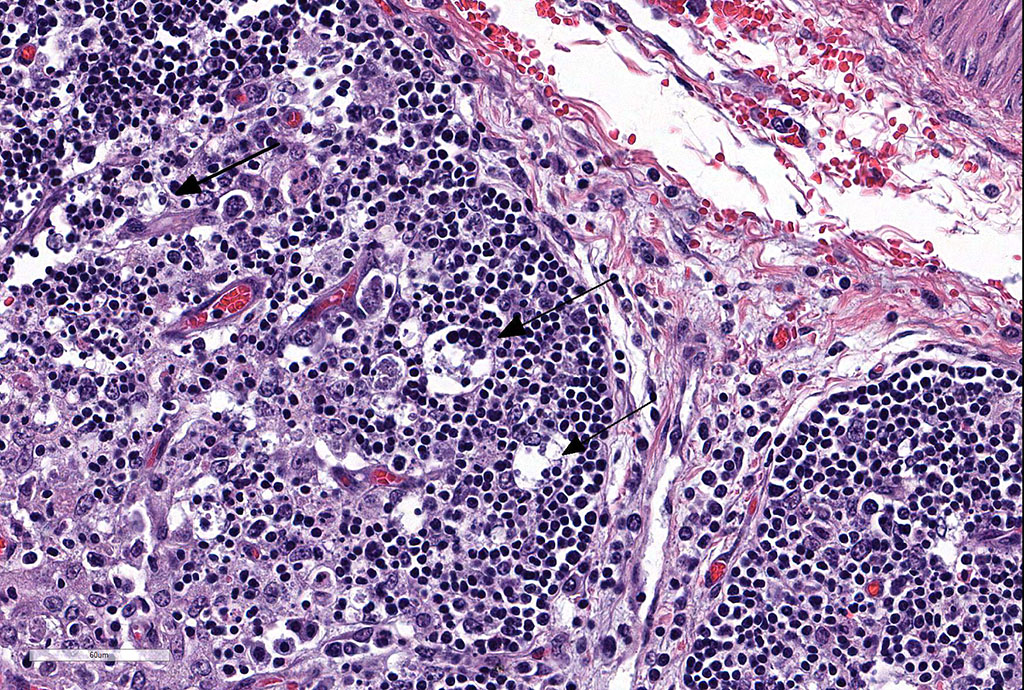
Thymus: There is marked depletion of cortical thymocytes affecting most if not all follicles, scattered foci of lymphocytolysis, marked histiocytic infiltration of the medulla and septa with occasional giant cell formation, and focal interlobular hemorrhages.
Heart: There are mild to moderate histiocytic infiltrates and fewer numbers of lymphocytes and plasma cells around small and medium caliber vessels throughout the epicardium, myocardium and epicardial fat.
Other findings: Mild to marked perivascular [vasculitis] and interstitial predominantly histiocytic infiltrates [fewer lymphocytes and plasma cells] in brain, lung, liver, kidney, spleen, lymph node, intestine, diaphragm, adrenal gland, and urinary bladder. There was marked lymphoid hyperplasia in spleen and lymph nodes in addition to infiltration by macrophages. Tissues were negative for Mycobacterium sp. on acid-fast stains.
Contributor’s Morphologic Diagnoses: Thymus: Thymitis, histiocytic to granulomatous, marked, diffuse with loss of cortical thymocytes and lymphocytolysis, consistent with epizootic bovine abortion.
Heart: Vasculitis, histiocytic and lymphoplasmacytic, multifocal, moderate.
Contributor’s Comment: Lesions are similar in submitted slides though there may be some variation in severity and extent. The clinical history, gross findings and microscopic lesions particularly those noted in the thymus, spleen and lymph nodes are considered characteristic of epizootic bovine abortion [EBA].
EBA, also known as foothill abortion, is a tick-born infection of cattle that results in late term abortions of fetuses, stillbirths, or the birth of weak calves. The name of the disease is misleading given that the disease is endemic, and distribution is confined to the range of the vector, the argasid tick Ornithodoros coriaceus, which includes foothills of California, and adjacent states of Nevada, Oregon and Northern Mexico.1,6,13 The tick is thought to transmit the agent from deer to cattle, and naïve cattle i.e. first time heifers or pregnant cows mainly beef cattle grazing for the first time in a tick-infested area, are primarily at risk.7,10 Infection from dam to fetus commonly occurs at 2-6 months gestation resulting in chronic disease in the fetus.7 The etiologic agent of EBA (aoEBA) has been identified as a novel organism in the class Deltaproteobacteria in the order Myxococcales. 10
Clinical findings include petechial hemorrhages of conjunctiva, tongue, oral mucous membranes and tracheal mucosa, palpable lymph nodes, and often ascites. Grossly there is splenomegaly, lymphadenomegaly, enlarged nodular liver, and enlarged thymus with hemorrhages.1,7,9 Microscopic lesions consist of marked lymphoid hyperplasia of lymph nodes and spleen accompanied by mononuclear, predominantly histiocytic, infiltration of medulla and sinuses. Histiocytes diffusely infiltrate medulla and septae of the thymus, and are present around vessels in multiple organs sometimes with giant cell formation.1,7,8,11 Bacterial rods, 1.5 to 3 µm long can often be seen in cytoplasm of histiocytes using Steiner’s silver stains, and there is positive cytoplasmic EBA immunohistochemical staining confined to foci of inflammation in multiple tissues.1
Diagnosis is based on compatible clinical history, gross and microscopic lesions, elevated fetal immunoglobulins particularly IgG, and exclusion of other agents commonly associated with bovine abortion.2,3. Supplemental diagnostic tests to identify agent in tissues include histochemical [Steiner’s silver] and immunohistochemical stains, 1 and TaqMan polymerase chain reaction.4,10
Successful transmission of the disease has been achieved by inoculation of thymus from EBA-positive fetuses in naïve pregnant cows12, and viability of the agent had been proved following serial passages and propagation of deltaproteobacterium in immunodeficient mice.2 While the tick O. coriaceus is identified as the known vector for the deltaproteobacterium, co-infection of the tick with Borrelia coriaceae has been reported, and antibody cross-reaction with Borrelia spp. in fetal tissues is possible.14
JPC Diagnosis: 1. Thymus: Thymitis, granulomatous, diffuse, marked, with marked lymphoid depletion.
- Heart: Pancarditis, granulomatous, diffuse, mild.
Conference Comment: Since the submission of this case in 2015, additional characterization of this agent and a name change has occurred. The organism is currently identified as Pajaroellobacter abortibovis, after the Pajaroello tick, (Ornithodorus coriaceous) which transmits the disease (and which was identified as the vector in 1976). The name “Pajaroello” (pa-har-way”-o) comes from the Spanish “paja” meaning straw and “huello” meaning the underside of a hoof, is also called to the grayback or leatherback tick, and is found only in California and Mexico extending from Humboldt County on the north to the Mexican isthmus of Tehuantepec.7
Phylogenetic characterization of this yet-to-be-cultured bacterium is currently based on analysis of the 16S ribosomal RNA gene, which places the bacterium in the order Myxococcales, suborder Sorangiineae, family Polyangiaceae and the bacterium is most closely related to Sorangium cellulosum. 5 Modified Gram staining, combined with transmission electron microscopy, provides strong evidence that the bacteria is gram-negative.5 PCR analysis has demonstrated P. abortibovis to be a myxobacterium a member of a family of gram-negative rods typically inhabiting soil. These bacteria are referred to as “gliding” bacteria as many produce a polysaccharide slime layer to aid in their motility.5 Its most closely related relative, S. cellulosum carries the largest genome of any known bacterium. In addition, its 16S ribosomal sequence establishes a distant connection to another deltaproteobacterium, Lawsonia intracellularis.5
Interestingly, the bite of the pajaroella tick (Ornithodoros coriaceous) has for many years been the subject of much misinformation and great myth. “A bite more severe to than that of the rattlesnake” is largely the product of the overactive imagination of the lay press, with hyperbolic early reports describing the bite as “intolerably sharp and painful… with intermittent irritation, irritability, and numbness” persisting for weeks to months. Local folklore in one area reported that “three bites would result in certain death.”7 The truth of the matter is that the macule or papule resulting from the bite of this tick is similar grossly and histologically to those of other ticks of a similar size.7
Contributing Institution:
California Animal Health and Food Safety Laboratory, University of California Davis, 105 W.
Central Avenue, San Bernardino, CA 92374 http://www.vetmed.ucdavis.edu/cahfs/
References:
- Anderson ML, Kennedy PC, Blanchard MT, et al. Histochemical and immunohistochemical evidence of a bacterium associated with lesions of epizootic bovine abortion. J Vet Diagn Invest 2006; 18:76–80.
- Blanchard MT, Chen C-I, Anderson M, et al. Serial passage of the etiologic agent of epizootic bovine abortion in immunodeficient mice. Vet Microbiol 2010; 144: 177-182.
- Blanchard MT, Anderson ML, Hoar BR et al. Assessment of a fluorescent antibody test for detection of antibodies against epizootic bovine abortion. J Vet Diagn Invest 2014; 26: 622-630.
- Brooks RS, Blanchard MT, Anderson ML, et al. Quantitiative duplex TaqMan real-time polymerase chain reaction for the assessment of the etiologic agent of epizootic bovine abortion. J Vet Diagn Invest 2011; 23: 1153 – 1159
- Brooks RD, Blanchard MT, Clothier KA, fish S, Anderson ML, Stott JL. Characterization of Pajaroellobacter abortibovis, the etiologic agent of epizootic bovine abortion. Vet Microbiol 2016; 192:73-80.
- Chen C-I, et al. Identification of the etiologic agent of epizootic bovine abortion in field-collected Ornithodoros coriaceus Koch ticks. Vet Microbiol 2007; 120:320-327.
- Failing RM, Lyon CB, McKittrick JE. The pajaroello tick bite – the frightening folklore and the mild disease. Calif Med 1972; 116(5):16-19.
- Foster, RA: Epizootic bovine abortion. In: Pathologic Basis of Veterinary Disease, 2007, McGavin MD, Zachary JF, eds., 4th, pp.1296, Elsevier, St. Louis, MO.
- Hall MR, Hanks D, Kvasnicka W et al. Diagnosis of epizootic bovine abortion in Nevada and identification of the vector. J Vet Diagn Invest 2002; 14: 205-210.
- King DP, Chen C-I, Blanchard MT, et al. Molecular identification of a novel deltaproteobacterium as the etiologic agent of epizootic bovine abortion (foothill abortion). J Clin Microbiol 2005; 43:604–609
- Schlafer DH, Miller RB, Epizootic bovine abortion In: Female genital system. Jubb Kennedy, and Palmer’sPathology of Domestic Animals, Maxie MG ed., 5th, vol 3, pp .510-512, Elsevier , Philadelphia, PA.
- Stott JL, Blanchard MT, Anderson M, et al. Experimental transmission of epizootic bovine abortion [foothill abortion]. Vet Microbiol 2002; 88: 161-173.
- Teglas MB, Drazenovich NL, Stott J, Foley JE: The geographic distribution of the putative agent of epizootic bovine abortion in the tick vector, Ornithodoros coriaceus. Vet Parasitol 2006; 140: 327-333.
- Teglas MB, Mapes S, Hodzic E, et al. Co-infection of Ornithodoros coriaceus with the relapsing fever spirochete Borrelia coriaceus, and the agent of epizootic bovine abortion. Med Vet Entom 2011; 25: 337-343.