Signalment:
14-month-old female mouse αB crystallin/HSPB2 knockout, C57Bl6 background (
Mus musculus).Genetically engineered mouse involved in ocular research relating to lens proteins. Three of three cagemates present with similar clinical signs, including hunched posture, rough haircoat, and poor body condition (body condition score 2/5). Marked kyphosis noted in all three mice. No improvement with supportive care over two days.
Gross Description:
Reduced subcutaneous, peritoneal, and reproductive fat deposits. Marked kyphosis with prominent dorsal deviation of the mid-thoracic spine. No other gross abnormalities detected.
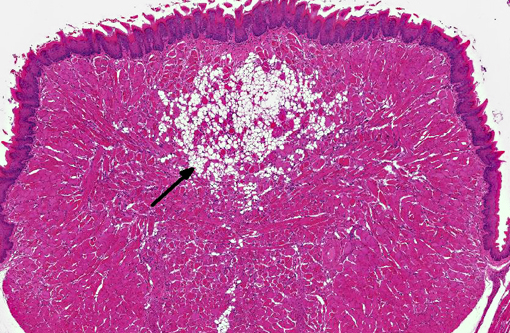
Histopathologic Description:
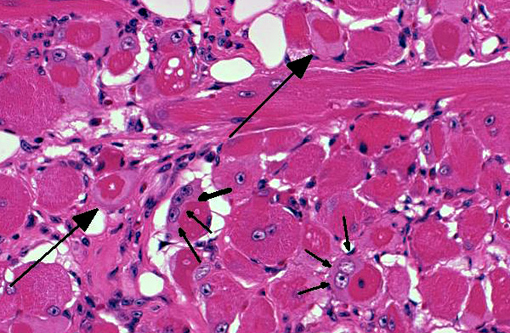
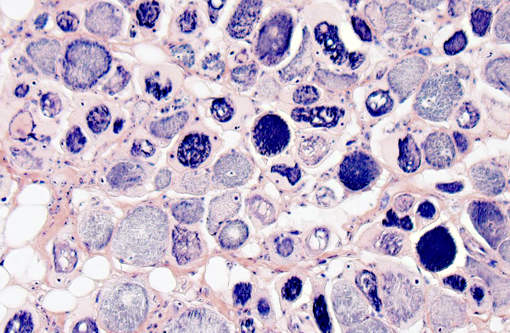
On decalcified cross sectional views of the head, there is a focal, marked loss of the normal muscle architecture of the posterior tongue characterized by a large central region of myofiber loss and replacement by white fat. Diffusely throughout the remainder of the tongue, there is moderate myofiber size variation, myofiber pallor and cytoplasmic vacuolation, and myofiber loss and replacement by perimyseal white fat and endomysial fibrous connective tissue. Moderate multifocal myofibers have uneven cellular staining with central strongly eosinophilic homogenous material (hyaline degeneration of the sarcoplasm). There is mild, multifocal lymphocytic and histiocytic inflammation. Other muscles of the head show less pronounced changes, although mild hyaline degeneration of the sarcoplasm, central nuclei, myofiber size variation, and perimyseal fibrosis are present in the muscles ventral to the bulla, the lateral muscles of the head, and the muscles of the hyoid apparatus.
There are moderate artifacts of processing and decalcification in the sections. Bilaterally, the lens is rotated, shrunken, fragmented, and there is artifactual retinal separation. Multifocal moderate speckles in the lens are due to contraction. One lens has mild focal ballooning and swelling of the peripheral lens fibers consistent with early cataractous change. The other lens has mild multifocal granular debris (liquefaction) and scattered Morgagnian globules containing eosinophilic material (aggregates of lens crystallins).Â
Morphologic Diagnosis:
Tongue: Moderate, multifocal to coalescing chronic myopathy with myodegeneration, fibrous and fatty connective tissue replacement, and hyaline degeneration of the sarcoplasm.Â
Lab Results:
Splenic culture: No aerobic or anaerobic growth.
Condition:
Crystallin mutation myopathy
Contributor Comment:
The αB-crystallin knockout mouse is a genetically engineered model with a targeted deletion of the αB-crystallin gene (and the nearby HSPB2 gene), created initially on a 129Sv background.(1) It has been proposed that αB-crystallin functions as a molecular chaperone for intermediate filament proteins.(3) Alpha B-crystallin and HSPB2 are stress -inducible heat shock proteins, and αB-crystallin is expressed predominantly in the lens but also in tissues such as cardiac and skeletal muscle.(1) These mice are viable and fertile and at approximately 40 weeks of age, they begin to develop skeletal muscle atrophy, kyphosis, osteoarthritis, and weight loss.(1) Myopathic changes in the tongue and axial musculature in αB-crystallin knockout mice which appear similar to inherited myofibrillar myopathies seen in humans have been reported.(1,2) Many human patients with dominant negative αB-crystallin mutations have an adult onset of the disease.(5,6) Axial muscles in the mice were examined and showed similar myopathic changes with marked fatty replacement and endomysial fibrosis and minimal associated inflammation. A predilection for tongue and truncal muscles has been previously reported in αB crystallinopathy; this predilection is thought to be secondary to increased expression of αB-crystallin in type I muscle fibers which are more abundant in axial musculature.(2) Trichrome staining was performed and highlights the perimyseal collagen deposition in this case. Additionally, mild to moderate cardiomyopathy (characterized by cardiomyocyte hyaline and vacuolar degeneration, variable fiber size, and interstitial fibrosis with mononuclear inflammation) was identified in all three mice examined in this case, which has not been previously reported in αB-crystallin knockout mice. Cardiomyopathy in human patients with αB-crystallin mutations is rarely reported. The presence of cardiomyopathy in the mice we examined may be related to genotype, background strain (increasing influence of C57BL6), or may represent an unrelated typical aging change in these mice. There are mild cataractous lens changes; however, significant artifact is present as these tissues were formalin fixed. Complete ocular phenotyping would require proper fixation and sectioning of the eyes.
JPC Diagnosis:
1. Skeletal muscle, tongue: Degeneration, necrosis and regeneration, focally extensive, marked, with fibrosis and fat infiltration.
2. Middle ear: Otitis media, suppurative, minimal.
3. Nasal cavity: Rhinitis, suppurative, minimal.
Conference Comment:
As stated by the contributor, αβ-crystallin is expressed primarily within the lens, as well as skeletal and cardiac muscle, where it is important for normal structure and function. Humans with αβ-crystallin mutations frequently present with cataracts, in addition to abnormal myofiber structure with subsequent myopathy.(1,5) The αB-crystallin knockout mouse was developed to study this rare human condition. In this case, lesions are confined to the tongue, while adjacent skeletal muscle appears relatively unaffected. Histochemical staining with Massons Trichrome highlights moderate collagen deposition surrounding degenerate lingual myofibers, while PTAH outlines the well-defined, deep blue-purple striations of myofibers in the adjacent, normal skeletal muscle. This is a significant contrast to degenerate muscle fibers within the tongue, which often lack striations and exhibit patchy, irregular uptake of PTAH.Â
In addition to the key histological features discussed above, the moderator noted the presence of rare accumulations of a homogenous, eosinophilic substance within the interstitium of the anterioventral nasal septum. Although participants were unable to demonstrate this material during conference, deposition of amyloid-like material within the nasal septum is a documented background finding commonly reported in aging mice. Its composition remains unidentified, however PAS positivity and a lack of birefringence with polarized light after Congo red staining suggests that the substance is not amyloid.(4)
References:
1. Brady JP, Garland DL, Green DE, et al. aB-Crystallin in lens development and muscle integrity: a gene knockout approach. IOVS. 2001;42:2924-2934.
2. Del Bigio MR, Chudley AE, Sarnat HB, et al. Infantile muscular dystrophy in Canadian aboriginals is an aB-Crystallinopathy. Ann Neurol. 2011;69:866-871.
3. Djabali K, de Nechaud B, Landon F, et al. AlphaB-crystallin interacts with intermediate filaments in response to stress. J Cell Sci. 1997;110: 2759-2769.
4. Haines DC, Chattopadhyay S, Ward JM. Pathology of aging B6;129 mice. Toxicol Pathol. 2001;29(6):653-661.
5. Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54:804-10.
6. Vicart P, Caron A, Guicheney P, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Gen. 1998;20:92-95.